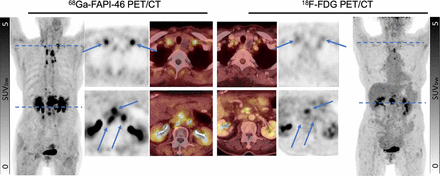
Visual Abstract
Abstract
Tumoral fibroblast activation protein expression is associated with proliferation and angiogenesis and can be visualized by PET/CT. We examined the prognostic value of [68Ga]Ga-fibroblast activation protein inhibitor (FAPI) (68Ga-FAPI)–46 PET/CT for different tumor entities in patients enrolled in 2 prospective imaging studies (NCT05160051, n = 30; NCT04571086, n = 115). Methods: Within 4 wk, 145 patients underwent 68Ga-FAPI-46 and [18F]FDG (18F-FDG) PET/CT. The association between overall survival (OS) and sex, age, tumor entity, total lesion number, highest SUVmax, and the presence of each nodal, visceral, and bone metastasis was tested using univariate Cox regression analysis. Multivariate analyses were performed for prognostic factors with P values of less than 0.05. Results: In the univariate analysis, shorter OS was associated with total lesion number and the presence of nodal, visceral, and bone metastases on 68Ga-FAPI-46 PET/CT (hazard ratio [HR], 1.06, 2.18, 1.69, and 2.05; P < 0.01, < 0.01, = 0.04, and = 0.02, respectively) and 18F-FDG PET/CT (HR, 1.05, 2.31, 1.76, and 2.30; P < 0.01, < 0.01, = 0.03, and < 0.01, respectively) and with SUVmax on 68Ga-FAPI-46 PET/CT (HR, 1.03; P = 0.03). In the multivariate analysis, total lesion number on 68Ga-FAPI-46 PET/CT was an independent risk factor for shorter OS (HR, 1.05; P = 0.02). In patients with pancreatic cancer, shorter OS was associated with total lesion number on 68Ga-FAPI-46 PET/CT (HR, 1.09; P < 0.01) and bone metastases on 18F-FDG PET/CT (HR, 31.39; P < 0.01) in the univariate analysis and with total lesion number on 68Ga-FAPI-46 PET/CT (HR, 1.07; P = 0.04) in the multivariate analyses. In breast cancer, total lesion number on 68Ga-FAPI-46 PET/CT (HR, 1.07; P = 0.02), as well as bone metastases on 18F-FDG PET/CT (HR, 9.64; P = 0.04), was associated with shorter OS in the univariate analysis. The multivariate analysis did not reveal significant prognostic factors. In thoracic cancer (lung cancer and pleural mesothelioma), the univariate and multivariate analyses did not reveal significant prognostic factors. Conclusion: Disease extent on 68Ga-FAPI-46 PET/CT is a predictor of short OS and may aid in future risk stratification by playing a supplemental role alongside 18F-FDG PET/CT.
In vivo visualization of fibroblast activation protein (FAP) by means of [68Ga]Ga-FAP inhibitor (FAPI) (68Ga-FAPI) PET/CT imaging is characterized by high tumor uptake and low background accumulation of radioligands (1). This results in high detection rates in a multitude of solid tumors in comparison with [18F]FDG (18F-FDG) PET/CT (2–4).
FAP expression has been confirmed in many cancers (90% of carcinomas), especially in the stroma in the tumor tissue, and thus may become a universal marker of cancer-associated fibroblasts (5). This expression has been associated with proliferation, invasion, angiogenesis, and drug resistance (5), leading to a poor prognosis in several malignancies, including gastric (5), colorectal (6), pancreatic (7), and non–small cell lung (8) cancer. However, only a few studies, mostly on small cohorts, have examined the prognostic value of 68Ga-FAPI PET/CT in this context (9–11).
To address this gap in knowledge, we compared the prognostic implications of 68Ga-FAPI-46 PET/CT and 18F-FDG PET/CT in a large population of patients with various tumors.
MATERIALS AND METHODS
Patients
We screened our institutional database of prospective imaging studies for consecutive patients who underwent 68Ga-FAPI-46 PET/CT and 18F-FDG PET/CT within 4 wk from April 2020 to September 2022 for imaging of tumors other than sarcoma (because of another ongoing project focused on sarcoma). The patient selection process is shown in Figure 1.
Consolidated Standards of Reporting Trials (CONSORT) diagram illustrating enrollment process. CECT = contrast-enhanced CT.
All patients gave written informed consent. Of these, 145 patients were included in 2 prospective imaging studies (NCT05160051, 30 interventional; NCT04571086, 115 observational). Data analysis was approved by the ethics committee of the University of Duisburg–Essen (20-9485-BO and 19-8991-BO). The patient subgroups have previously been reported (12–16). We obtained the precursor of 68Ga-FAPI-46 from SOFIE Biosciences.
Image Acquisition
At 23.3 ± 20.2 min (range, 9–102 min) after the injection of 123.9 ±31.0 MBq (range, 60–199 MBq) of 68Ga-FAPI-46, PET/CT was performed on a Siemens 128-slice Biograph mCT (26/145 patients, 17.9%), Siemens Biograph Vision (115/145 patients, 79.3%), or Philips Vereos (4/145 patients, 2.8%). Acquisition times were based on a prior publication by our group (13).
18F-FDG PET/CT was performed 71.8 ±18.2 min (range, 43–147 min) after the injection of 267.1 ± 84.6 MBq (range, 94–458 MBq) of 18F-FDG. Images were acquired on a Biograph mCT (27/145 patients, 18.6%), Biograph Vision (109/145 patients, 75.2%), or Vereos (9/145 patients, 6.2%). All PET images were iteratively reconstructed with time of flight (Biograph mCT: 3 iterations and 21 subsets, gaussian filtering of 4 mm; Biograph Vision: 4 iterations and 5 subsets, gaussian filtering of 2 mm; Vereos: 2 iterations and 10 subsets, gaussian filtering of 4 mm).
Image Interpretation and Quantitative Analysis
Images were interpreted by a board-certified nuclear medicine physician and radiologist with 14 y of experience, who had completed institutional reader training on 50 68Ga-FAPI-46 PET/CT datasets including common pitfalls. The reader was not aware of the clinical information. Masked interpretation was chosen to avoid biases due to knowledge of clinical information and to measure the standalone impact of the imaging modalities, even though lack of clinical information may trigger faulty image interpretation at times.
Lesions were classified as malignant if they exhibited focal tracer accumulation incongruent with physiologic or nonneoplastic uptake (17) and were categorized into the following anatomic regions: primary, cervicothoracic nodal metastases, abdominopelvic nodal metastases, pulmonary metastases, hepatic metastases, other visceral metastases, and bone metastases. Lesion number (≤10 per region to avoid individual bias, from larger to smaller lesions), and SUVmax was assessed visually on Syngo.via software (Siemens Healthineers). Representative diagnosis cases are shown in Figures 2 and 3.
Intraindividual comparison between 68Ga-FAPI-46 PET/CT and 18F-FDG PET/CT for restaging in patient with postoperative pancreatic head cancer (73-y-old woman with extensive nodal metastases). Bilateral nodal metastases in supraclavicular region were detectable only on 68Ga-FAPI-46 PET/CT (SUVmax, 5.74 on right side and 10.19 on left side; arrows); findings on 18F-FDG PET/CT were nonspecific (SUVmax, 2.93 on right side and 3.58 on left side; arrows). At level of bilateral renal pelvis, there were 3 and only 1 detectable paraaortic nodal metastases on 68Ga-FAPI-46 PET/CT (SUVmax, 6.75, 11.37, and 11.75 from right to left) and 18F-FDG PET/CT (SUVmax, 7.94; other 2 lymph nodes not measurable), respectively (arrows). SUVbw = SUV based on body weight.
Intraindividual comparison between 68Ga-FAPI-46 PET/CT and 18F-FDG PET/CT for restaging in patient with postoperative left breast cancer (36-y-old woman with large number of metastases). Several metastases to muscles were detectable only on 18F-FDG PET/CT (red arrows). At same axillary level (right side of body), there were 1 and 3 detectable nodal metastases on 68Ga-FAPI-46 PET/CT (SUVmax, 3.47; other 2 distal lymph nodes not measurable) and on 18F-FDG PET/CT (SUVmax, 26.28, 18.54, and 29.60 from proximal to distal lymph nodes), respectively (blue arrows). In liver region (lower part of images), 2 liver metastases were detectable on 18F-FDG PET/CT (SUVmax, 6.65 in segment 4 and 4.50 in segment 5); however, no liver metastases were detectable on 68Ga-FAPI-46 PET/CT (blue arrows). SUVbw = SUV based on body weight.
Statistical Analysis
Overall survival (OS) was defined as the interval from the day of the PET/CT scans (68Ga-FAPI-46 PET/CT and 18F-FDG PET/CT) until death or the end of the study (censored in June 2023). For OS, we performed univariate Cox proportional hazards regression analysis using the following variables: sex, age, restaging (vs. initial staging), tumor entity, total lesion number, the presence of nodal metastases, the presence of visceral metastases, the presence of bone metastases, and the highest SUVmax of all lesions. Prognostic factors with a P value of less than 0.05 in the univariate analysis, as well as the tumor entity as a categoric parameter (considering the heterogeneity of tumor characteristics), were considered statistically significant and tested in multivariate analyses. We also performed subanalyses for the patients with pancreatic cancer, breast cancer, and thoracic cancer (lung cancer and pleural mesothelioma). Separate Cox analyses for each tumor entity (pancreatic cancer, breast cancer, and thoracic cancer) are susceptible to multiple-comparison problems due to small sample sizes. To resolve this issue, we entered into the multivariate Cox analysis only the prognostic parameters that were significant predictors of OS in the multivariate analysis on the entire cohort. We performed Kaplan–Meier analysis using log-rank testing to determine the statistical association between OS and findings on 18F-FDG PET/CT and 68Ga-FAPI-46 PET/CT. For statistical analysis, we used MedCalc version 22.007, 32-bit (MedCalc Software), and Prism 8 (GraphPad Software). Numeric values are provided as mean ± SD.
RESULTS
Patient Cohort
The final cohort included 145 patients, of whom 85 were male and 60 were female (mean ± SD, 61.6 ± 11.8 y old; range, 30–85 y old). We enrolled 53 patients (36.6%) for staging and 92 patients (63.4%) for restaging. The most common tumor entities were pancreatic cancer (n = 40), mesothelioma (pleural, n = 18; peritoneal, n = 2), and breast cancer (n = 17). Sixty-four of 145 (44.1%) patients died during the mean follow-up period of 13.8 mo (range, 1–30 mo). Patient characteristics are provided in Table 1. Because we enrolled patients requiring either staging or restaging, we classified only the patients with T(positive)/N0/M0, T(any)/N(positive)/M0, and T(any)/N(any)/M(positive), by referring to the 68Ga-FAPI-46 PET/CT, 18F-FDG PET/CT, and contrast-enhanced CT, all of which were performed within 4 wk. The median total lesion number was 4 on 68Ga-FAPI-46 PET/CT (range, 0–53; 1 with no lesions; 44 with 1 lesion; 27 with 2 or 3 lesions; 8 with 4 or 5 lesions; 20 with 6–10 lesions; 45 with >10 lesions) and 3 on 18F-FDG PET/CT (range, 0–57; 8 with no lesions; 47 with 1 lesion; 26 with 2 or 3 lesions; 7 with 4 or 5 lesions; 20 with 6–10 lesions; 37 with >10 lesions).
Patient Characteristics (n = 145)
The treatment records were available for 133 of 145 patients (91.7%). Ninety-four of 145 patients (64.8%) underwent surgery. In 93 cases (93/145, 64.1%), the primary was resected, and in 22 cases (22/145, 15.2%), metastases were resected; in 21 of those, both the primary and metastases were resected (21/145, 14.5%).
Of the 94 patients who underwent surgery, 79 (79/145, 54.5%) received systemic therapy (chemotherapy or immunotherapy). Of the remaining 39 recorded patients without surgery (39/145, 26.9%), 31 (31/145, 21.4%) received systemic therapy. Systemic therapy was used in 110 of 145 (75.9%) patients, of whom 14 had breast cancer, 5 had lung cancer, 14 had pleural mesothelioma, 2 had peritoneal mesothelioma, 13 had cholangiocellular cancer, 33 had pancreatic cancer, 13 had colorectal cancer, 9 had renal cell cancer, and 7 had prostate cancer. Other therapies consisted of radiopharmaceutical therapy with [177Lu]Lu-PSMA-617 (n = 4), radioembolization (n = 3), [223Ra]Ra-chloride (n = 1), hormone therapy (for breast cancer and prostate cancer, n = 11), radiofrequency ablation (n = 2), and external-beam radiotherapy (n = 38). Information on the treatment is summarized in Table 1.
Prognostic Analysis
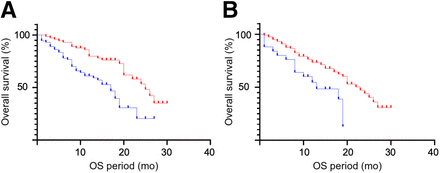
In the univariate analysis for 68Ga-FAPI-46 PET/CT, total lesion number; the presence of nodal, visceral, and bone metastases; and the highest SUVmax of all lesions were significant predictors of short OS (hazard ratio [HR], 1.06, 2.18, 1.69, 2.05, and 1.03, respectively; 95% CI, 1.03–1.08, 1.32–3.60, 1.03–2.77, 1.12–3.77, and 1.00–1.07, respectively; P < 0.01, < 0.01, = 0.04, = 0.02, and = 0.03, respectively; Table 2). In the multivariate analysis (Table 2), total lesion number was significantly associated with OS (HR, 1.05; 95% CI, 1.01–1.10; P = 0.02), whereas the presence of nodal, visceral, and bone metastases and the highest SUVmax of all lesions were not (HR, 1.12, 1.10, 2.21, and 1.02, respectively; 95% CI, 0.57–2.19, 0.56–2.16, 0.93–5.26, and 0.98–1.07, respectively; P = 0.75, 0.78, 0.07, and 0.29, respectively). Kaplan–Meier curves for OS based on the total lesion number are shown in Figure 4 using the median total lesion number (n = 4) as the cutoff. The median survival of patients with at least 4 lesions (73 patients) versus less than 4 lesions (72 patients) was 17 and 25 mo, respectively (P < 0.001).
Uni- and Multivariate Analyses of OS Using Cox Proportional Hazards Regression Analysis (Death Events, n = 64; Censored, n = 81)
Kaplan–Meier analyses for OS regarding total lesion number on 68Ga-FAPI-46 PET/CT (A) and presence of bone metastases on 18F-FDG PET/CT (B). Blue and red lines are groups with ≥4 total lesions (median value of all patients) and those with ≤3 total lesions in A (P < 0.001) and group with positive bone metastases and those with negative bone metastases in B (P < 0.005), respectively.
Regarding 18F-FDG PET/CT (Table 2), total lesion number and the presence of nodal, visceral, and bone metastases were significantly associated with shorter OS (HR, 1.05, 2.31, 1.76, and 2.30, respectively; 95% CI, 1.03–1.08, 1.41–3.80, 1.07–2.92, and 1.27–4.17, respectively; P < 0.01, < 0.01, = 0.03, and < 0.01, respectively). In the multivariate analysis (Table 2), the presence of bone metastases was a predictor of shorter OS (HR, 3.46; 95% CI, 1.49–8.03; P < 0.01), whereas total lesion number and the presence of nodal and visceral metastases were not (HR, 1.04, 1.53, and 1.20, respectively; 95% CI, 0.999–1.08, 0.81–2.88, and 0.60–2.39, respectively; P = 0.055, 0.19, and 0.61, respectively). Kaplan–Meier curves for OS in the group with positive bone metastases (25 patients) and those with negative bone metastases (120 patients) are shown in Figure 4. The median survival was 13 and 23 mo, respectively (P < 0.005).
Regarding 68Ga-FAPI-46 PET/CT in the patients with pancreatic cancer, total lesion number was significantly associated with shorter OS in the univariate analysis (HR, 1.09; 95% CI, 1.03–1.15; P < 0.01) and in the multivariate analysis (HR, 1.07; 95% CI, 1.004–1.13; P = 0.04). For 18F-FDG PET/CT, bone metastases were a significant prognostic indicator for shorter OS in the univariate analysis (HR, 31.39; 95% CI, 4.33–227.36; P < 0.01). In the multivariate analysis, bone metastases were borderline-significant (HR, 8.67; 95% CI, 0.91–82.78; P = 0.06). These results are summarized in Table 3.
Uni- and Multivariate Subanalyses of OS for Patients with Pancreatic Cancer Using Cox Proportional Hazards Regression Analysis (Death Events, n = 28; Censored, n = 12)
As for the patients with breast cancer using 68Ga-FAPI-46 PET/CT, total lesion number was significantly associated with shorter OS in the univariate analysis (HR, 1.07; 95% CI, 1.01–1.13; P = 0.02) but was not significant in the multivariate analysis (HR, 1.03; 95% CI, 0.95–1.11; P = 0.47). For 18F-FDG PET/CT, bone metastases were a significant prognostic indicator for OS in the univariate analysis (HR, 9.64; 95% CI, 1.12–83.30; P = 0.04) but was not significant in the multivariate analysis (HR, 5.58; 95% CI, 0.38–81.50; P = 0.21). These results are summarized in Table 4.
Uni- and Multivariate Subanalyses of OS for Patients with Breast Cancer Using Cox Proportional Hazards Regression Analysis (Death Events, n = 6; Censored, n = 11)
Concerning the subanalysis for patients with thoracic cancer, including lung cancer and pleural mesothelioma, no PET-derived prognostic factors were not associated with OS in the uni- and multivariate analyses. These results are summarized in Table 5.
Uni- and Multivariate Subanalyses of OS for Patients with Thoracic Cancer Using Cox Proportional Hazards Regression Analysis (Death Events, n = 11; Censored, n = 19)
DISCUSSION
The results of our study reveal that 68Ga-FAPI-46 PET/CT–based parameters have prognostic value in a mixed population of cancer patients. The presence of bone metastases on 18F-FDG PET/CT, and lesion number on 68Ga-FAPI-46 PET/CT (HR, 1.05), were independent risk factors for shorter OS in the multivariate analysis. Although the HR of the latter may appear small, continuous variables with a wide range (0–53) often display lower HRs. Yet the effect of lesion number on the extreme ends of the spectrum is not negligible, as shown in the context of Ki-67, an established prognostic parameter in a multitude of malignancies, where HRs in the similar range are commonly observed (e.g., 1.05 in patients with adrenocortical carcinoma regarding OS (18)). In addition, the univariate analysis identified metastases to nodes, visceral organs, and bone and the highest SUVmax of all lesions on 68Ga-FAPI-46 PET/CT as significant prognostic indicators for shorter OS. 68Ga-FAPI-46 PET/CT–based parameters such as total lesion number, and 18F-FDG PET/CT–based parameters such as the presence of hypermetabolic bone metastases, may aide risk stratification alongside other, already-established, prognostic markers (19). 18F-FDG PET/CT as a prognostic marker is well established in a multitude of malignancies, underpinned by an extensive body of research, and is well understood, especially with regard to its associations with dedifferentiation and proliferation. 18F-FDG PET/CT–derived markers that have been well studied in their association with OS are, among others, metabolic tumor volume and total lesion glycolysis, such as in patients with lung, pancreatic, and breast cancer (19–21).
On the basis of our results, it appears unlikely that 68Ga-FAPI-46 PET/CT–derived markers will generally replace 18F-FDG PET/CT, yet they may serve as complementary markers, with lesion number being particularly promising. The latter may be the consequence of a better diagnostic performance in some tumor entities (2,4,11). Additionally, the identification of PET biomarkers derived from 68Ga-FAPI-46 PET/CT can be of particular interest in tumor entities, where it could eventually become the gold standard for PET imaging (22).
68Ga-FAPI-46 PET/CT may therefore aid treatment decisions not just by providing accurate staging but also by providing prognostic information. The impact on patient prognosis has previously been shown in the context of patients with colorectal cancer, where FAP expression on 68Ga-FAPI-46 PET/CT was associated with a significantly shorter relapse-free survival (9).
In our subanalysis of patients with pancreatic cancer, bone metastases (HR, 31.39; 95% CI, 4.33–227.36; P < 0.01) in 18F-FDG PET/CT were significantly associated with shorter OS in the univariate analysis and showed borderline significance (HR, 8.67; 95% CI, 0.91–82.78; P = 0.06) in the multivariate analysis. On the other hand, with regard to 68Ga-FAPI-46 PET/CT–derived parameters, only total lesion number reached statistical significance in the univariate analysis (HR, 1.09; 95% CI, 1.03–1.15; P < 0.01) and multivariate analysis (HR, 1.07; 95% CI, 1.004–1.13; P = 0.04).
A prior study on pancreatic cancer has shown that SUVmax in 68Ga-FAPI-04 PET/CT had a significant independent prognostic value for recurrence-free survival and that total pancreatic FAP expression (the sum of the multiplication of SUVmean and total FAPI-avid volume) was a significant prognostic indicator for OS (10). Similarly, in a published metaanalysis, tumor SUVmax on 18F-FDG PET/CT has been shown to be a significant prognostic factor for OS (19). Furthermore, a high glycolytic activity in pancreatic cancer has been linked with subtypes that commonly exhibit a poor prognosis (e.g., basal subtype) and is associated with metastatic spread (23). In our study, we additionally found that total lesion number on 68Ga-FAPI-46 PET/CT could be a useful prognostic factor for OS in patients with pancreatic cancer. Importantly, the presence of bone metastases on PET/CT correlated more strongly with OS for 18F-FDG than for 68Ga-FAPI-46 in our study. This may be partly attributable to the fact that of the 4 of 39 patients with bone metastases secondary to pancreatic cancer, bone metastases were detected by 18F-FDG PET/CT, by 68Ga-FAPI-46 PET/CT, and by both modalities in 2, 3, and 1 cases, respectively. The large discrepancy in HR between the 2 modalities may therefore be caused by the low number of positive cases in the subgroup with pancreatic cancer.
To our knowledge, this was the first study to report on the prognostic implications of FAPI PET/CT in patients with breast cancer:
In our cohort, total lesion number on 68Ga-FAPI-46 PET/CT, and bone metastases on 18F-FDG PET/CT, were significant prognostic indicators for shorter OS in the univariate analysis; however, total lesion number on 68Ga-FAPI-46 PET/CT and bone metastases on 18F-FDG PET/CT were not significant in the multivariate analysis. The prognostic value of 18F-FDG PET/CT has been shown by an expansive body of evidence, for which a correlation between glycolytic activity on the one hand and tumor aggressiveness and poor prognosis on the other hand could be established (20,24). The lack of statistically significant results in the multivariate analysis may at least partially be attributable to insufficient statistical power due to the sample size.
A central limitation of this study is the low number of patients for each tumor entity, potentially affecting statistical power and calling for further prospective analyses on larger cohorts. Especially for the Cox subanalysis, there are few total events and few events per variable (25). Also, it has yet to be determined which PET-derived parameters can most accurately predict OS. Future multicenter prospective analyses with a larger sample size may be warranted to confirm the results. In addition, the 50 cases used to train the reader may be a limitation, since interobserver agreement has been shown to be moderate at this experience level; an experience level of at least 300 cases may be needed for substantial agreement (26). The prognostic parameters we assessed could complement risk stratification alongside already-established risk factors, such as resection status and neural invasion in the context of pancreatic cancer (27,28). Also, the absence of segmentation of PET-derived whole-body tumor volume and whole-body SUVmean may be a major limitation.
CONCLUSION
Here, we demonstrate an association of disease extent (parametrized by total lesion number on 68Ga-FAPI-46 PET/CT) on the one hand and OS on the other hand in various malignancies, such as pancreatic cancer. In line with prior publications, 18F-FDG PET/CT allowed for stratification of prognosis, especially in the presence of bone metastases. Improved risk stratification may aid patient management in the future.
DISCLOSURE
Masao Watanabe obtained a postdoctoral fellowship from Humboldt Foundation. Wolfgang Fendler reports fees from SOFIE Biosciences (research funding), Janssen (consultant, speaker), Calyx (consultant, image review), Bayer (consultant, speaker, research funding), Novartis (speaker, consultant), Telix (speaker), GE Healthcare (speaker), Eczacıbaşı Monrol (speaker), Abx (speaker), Amgen (speaker), and Urotrials (speaker), outside the submitted work. Rainer Hamacher reports grants from the Clinician Science Program of the University Medicine Essen Clinician Scientist Academy. He also reports other support from Eli Lilly and Company, Novartis, and PharmaMar, as well as personal fees from PharmaMar and Eli Lilly and Company, outside the submitted work. Kim Pabst reports personal fees and research funds from Bayer Healthcare, outside the submitted work, as well as a Junior Clinician Scientist Stipend from the University Medicine Essen Clinician Scientist Academy and travel fees from IPSEN. Stefan Kasper received honoraria from Merck Serono, MSD, Novartis, BMS, Amgen, Roche, Sanofi-Aventis, Servier, Incyte, Pierre Fabre, and Lilly and research funding from Lilly, BMS, and Roche. Bastian von Tresckow is an advisor or consultant for Allogene, Amgen, BMS/Celgene, Cerus, Gilead Kite, Incyte, IQVIA, Lilly, Merck Sharp & Dohme, Miltenyi, Novartis, Noscendo, Pentixapharm, Pfizer, Pierre Fabre, Qualworld, Roche, Sobi, and Takeda; has received honoraria from AbbVie, AstraZeneca, BMS/Celgene, Gilead Kite, Incyte, Lilly, Merck Sharp & Dohme, Novartis, Roche Pharma AG, and Takeda; reports research funding from Esteve (to the institution), Merck Sharp & Dohme (to the institution), Novartis (to the institution), and Takeda (to the institution); and reports travel support from AbbVie, AstraZeneca, Gilead Kite, Lilly, Merck Sharp & Dohme, Pierre Fabre, Roche, Takeda, and Novartis, all outside the submitted work. Sherko Kümmel reports a consultant or advisory role with Roche/Genentech, Genomic Health, Novartis, AstraZeneca, Amgen, Celgene, SOMATEX, Daiichi Sankyo, pfm medical, Pfizer, MSD Oncology, Lilly, Sonoscape, Gilead Sciences, Seagen, and Agendia. He also reports travel support and expenses from Roche, Daiichi Sankyo, and Gilead Sciences and an uncompensated relationship with WSG. Boris Hadaschik declares grants to the institution from Novartis, BMS, and the German Research Foundation; consulting fees from ABX, Accord, Amgen, AstraZeneca, Bayer, BMS, Janssen, Lightpoint Medical, and Pfizer; payment for lectures from Accord, Astellas, Janssen, and Monrol; support for travel or attending meetings from Bayer, Ipsen, and Janssen; and participation on data safety monitoring boards for Janssen, all outside the submitted work. Viktor Grünwald has received honoraria from Bristol-Myers Squibb, Pfizer, Ipsen, Eisai, MSD Oncology, Merck HealthCare, EUSAPharm, Apogepha, and Ono Pharmaceutical; has an advisory role with Bristol-Myers Squibb, Pfizer, MSD Oncology, Merck HealthCare, Ipsen, Eisai, Debiopharm, PCI Biotech, Cureteq, and Oncorena; and receives travel funds from Pfizer, Ipsen, and Merck HealthCare. Jens Siveke reports honoraria from AstraZeneca, Bayer, Immunocore, Novartis, Roche/Genentech, and Servier, outside the submitted work. Work in his laboratory is supported by the German Cancer Consortium. His institution receives research funding from Bristol-Myers Squibb, Celgene, Eisbach, Bio, and Roch/Genentech. He holds ownership in and serves on the board of directors of Pharma15. Ken Herrmann reports personal fees from Bayer, SIRTEX, Adacap, Curium, Endocyte, IPSEN, Siemens Healthineers, GE Healthcare, Amgen, Novartis, ymabs, Aktis Oncology, Theragnostics, and Pharma 15; personal fees and other fees from SOFIE Biosciences; nonfinancial support from ABX; and grants and personal fees from BTG, outside the submitted work. Manuel Weber reports personal fees from Boston Scientific, Terumo, Advanced Accelerator Applications, IPSEN, and Eli Lilly, outside the submitted work. No other potential conflict of interest relevant to this article was reported.
KEY POINTS
QUESTION: Do findings from 68Ga-FAPI-46 PET/CT have prognostic implications regarding OS?
PERTINENT FINDINGS: Disease extent derived from 68Ga-FAPI-46 PET/CT is a predictor of OS and may enhance risk stratification in various solid tumors.
IMPLICATIONS FOR PATIENT CARE: Improved tumor detection and risk stratification may aide clinical decisions and the pursuit of personalized medicine.
Footnotes
Published online May 23, 2024.
- © 2024 by the Society of Nuclear Medicine and Molecular Imaging.
REFERENCES
- Received for publication October 31, 2023.
- Accepted for publication May 7, 2024.