Visual Abstract
Abstract
Tissue-resident macrophages are complementary to proinflammatory macrophages to promote the progression of atherosclerosis. The noninvasive detection of their presence and dynamic variation will be important to the understanding of their role in the pathogenesis of atherosclerosis. The goal of this study was to develop a targeted PET radiotracer for imaging CD163-positive (CD163+) macrophages in multiple mouse atherosclerosis models and assess the potential of CD163 as a biomarker for atherosclerosis in humans. Methods: CD163-binding peptide was identified using phage display and conjugated with a NODAGA chelator for 64Cu radiolabeling ([64Cu]Cu-ICT-01). CD163-overexpressing U87 cells were used to measure the binding affinity of [64Cu]Cu-ICT-01. Biodistribution studies were performed on wild-type C57BL/6 mice at multiple time points after tail vein injection. The sensitivity and specificity of [64Cu]Cu-ICT-01 in imaging CD163+ macrophages upregulated on the surface of atherosclerotic plaques were assessed in multiple mouse atherosclerosis models. Immunostaining, flow cytometry, and single-cell RNA sequencing were performed to characterize the expression of CD163 on tissue-resident macrophages. Human carotid atherosclerotic plaques were used to measure the expression of CD163+ resident macrophages and test the binding specificity of [64Cu]Cu-ICT-01. Results: [64Cu]Cu-ICT-01 showed high binding affinity to U87 cells. The biodistribution study showed rapid blood and renal clearance with low retention in all major organs at 1, 2, and 4 h after injection. In an ApoE−/− mouse model, [64Cu]Cu-ICT-01 demonstrated sensitive and specific detection of CD163+ macrophages and capability for tracking the progression of atherosclerotic lesions; these findings were further confirmed in Ldlr−/− and PCSK9 mouse models. Immunostaining showed elevated expression of CD163+ macrophages across the plaques. Flow cytometry and single-cell RNA sequencing confirmed the specific expression of CD163 on tissue-resident macrophages. Human tissue characterization demonstrated high expression of CD163+ macrophages on atherosclerotic lesions, and ex vivo autoradiography revealed specific binding of [64Cu]Cu-ICT-01 to human CD163. Conclusion: This work reported the development of a PET radiotracer binding CD163+ macrophages. The elevated expression of CD163+ resident macrophages on human plaques indicated the potential of CD163 as a biomarker for vulnerable plaques. The sensitivity and specificity of [64Cu]Cu-ICT-01 in imaging CD163+ macrophages warrant further investigation in translational settings.
Cardiovascular disease is the leading cause of death worldwide, with atherosclerosis being the primary cause (1,2). It is known that macrophages play important and diverse roles in the initiation, progression, and complication of atherosclerosis (3), thus making macrophages not only diagnostic biomarkers to assess the progression and vulnerability of plaques (4–7) but also targets in drug development for pharmacologic intervention to modulate their activities in atherosclerosis and other inflammatory diseases (8–12).
Transformation of different phenotypes of macrophages regulates the initiation, development, and cessation of inflammatory diseases (13). Thus, it is critical to track the well-defined macrophage populations that have constant expression of surface markers across multiple organs to interrogate their temporal–spatial distribution along with the progression and regression of inflammatory diseases (14–16). Many molecular imaging agents have been developed to detect specific biomarkers (e.g., C-C motif chemokine receptor 2 and CXC motif chemokine receptor 4) upregulated on macrophages to assess the vulnerability of atherosclerotic lesions (7,17–23). Although some of these agents have shown promising results in patients with atherosclerosis, their potential to predict the rupture of plaques has not been realized (23–25). The noninvasive and accurate identification of patients with high-risk plaques who may benefit from immunomodulatory therapies is still an unmet clinical need.
CD163 is a 130-kDa transmembrane scavenger receptor expressed almost exclusively by resident macrophages. In contrast to the infiltrating monocyte-derived macrophages that often accumulate in response to local inflammatory cues in the specific tissue, resident macrophages are seeded during embryonic development and reside in many tissues, including liver and brain (14). The CD163-positive (CD163+) macrophages play an important role in tissue homeostasis and serve as a first-line defender against pathogen invasion because of their antiinflammatory functions (11). Moreover, CD163 is known as the exclusive receptor to clear the hemoglobin–haptoglobin complex on intravascular hemolysis. This leads to a distinct phenotype of macrophage termed M(Hb) found in areas of neoangiogenesis and hemorrhage, characterized by high expression of CD163 and reduced proinflammatory cytokine production, which distinguish this type of macrophage from foamy macrophages (26–28). Given the role of intraplaque hemorrhage in plaque destabilization (29), CD163+ macrophage expression is associated with plaque vulnerability in human carotid plaques and is significantly higher in symptomatic patients than in the asymptomatic population, highlighting its potential as a biomarker for vulnerable plaques (26,30).
CD163-targeting radiotracers have been previously reported. However, the in vivo imaging studies were few and showed suboptimal targeting efficiency (31,32). Therefore, we performed phage display to screen and identify a CD163-targeting peptide (ICT-01) using human CD163 protein (33). We conjugated a 2-[1,4,7-triazacyclononan-1-yl-4,7-bis(tBu-ester)]-1,5-pentanedioic acid (NODAGA) chelator to the peptide and radiolabeled with 64Cu ([64Cu]Cu-ICT-01). The in vitro binding affinity of [64Cu]Cu-ICT-01 was determined in CD163-overexpressing U87 cells and RAW264.7 cells (34,35). Pharmacokinetic evaluation revealed rapid renal clearance of [64Cu]Cu-ICT-01. In multiple murine atherosclerosis models, [64Cu]Cu-ICT-01 demonstrated sensitive and specific detection of CD163, which was further confirmed by ex vivo tissue characterization. In human carotid plaque specimens, CD163+ macrophages were highly expressed and associated with intraplaque hemorrhage, indicating the potential of CD163 as a biomarker of high-risk plaque. Moreover, [64Cu]Cu-ICT-01 showed specific detection of human CD163+ macrophages, suggesting a potential translational pathway.
MATERIALS AND METHODS
Full materials and methods with detailed descriptions are provided in the supplemental materials (available at http://jnm.snmjournals.org).
In the statistical analysis, group variation is described as mean ± SD. Groups were compared using 1-way ANOVA with a Tukey adjustment using Prism (version 10.0.2; GraphPad). Individual group differences were determined using a 2-tailed Mann–Whitney test. The significance level in all tests was a P value of less than 0.05.
RESULTS
Synthesis, In Vitro Cell-Binding Assay, and Biodistribution
The ICT-01 conjugate was characterized with high-performance liquid chromatography and electrospray ionization mass spectrometry, which showed chemical purity and the conjugation of 1 NODAGA chelator per ICT-01 peptide (Supplemental Fig. 1). Radiolabeling with 64Cu showed high specific activity ([64Cu]Cu-ICT-01, 111–185 MBq/nmol), enabling trace amount administration for in vivo imaging. The mouse serum stability study demonstrated that the tracer was 100% stable during the 1-h incubation. The blood metabolism study showed that [64Cu]Cu-ICT-01 was stable (∼92%) in vivo at 1 h (Supplemental Fig. 2). The [64Cu]Cu-ICT-01 in vitro binding assay in human U87 and mouse RAW 264.7 cells showed specific and high binding affinity, with half-maximal inhibitory concentrations of 14.4 ± 1.0 nM and 28.2 ± 13.4 nM, respectively (Fig. 1A, n = 3).
In vitro and in vivo characterization of [64Cu]Cu-ICT-01. (A) Cell-binding assay in human U87 cells and mouse RAW 264.7 cells. (B) Biodistribution in WT C57BL/6 mice (n = 4/group). IC50 = half-maximal inhibitory concentration.
In vivo pharmacokinetic evaluation of [64Cu]Cu-ICT-01 was performed on wild-type (WT) C57BL/6 mice at 1, 2, and 4 h after injection via the tail vein. As shown in Figure 1B, [64Cu]Cu-ICT-01 demonstrated effective renal and blood clearance leading to low retention in most organs. Both blood and heart had less than 0.5 percentage injected dose (%ID)/g retention at all time points. Hepatic uptake was always less than 2 %ID/g at the 4-h collection, and a similar pattern was observed for the intestine. In the spleen, radiotracer uptake was consistently below 0.5 %ID/g at all time points. Additionally, [64Cu]Cu-ICT-01 biodistribution blocking studies were performed at 1 h after injection (Supplemental Fig. 3). The significantly decreased uptake in liver, spleen, and thymus indicated its targeting specificity.
CD163 PET in ApoE−/− and Ldlr−/− Mouse Atherosclerosis Models
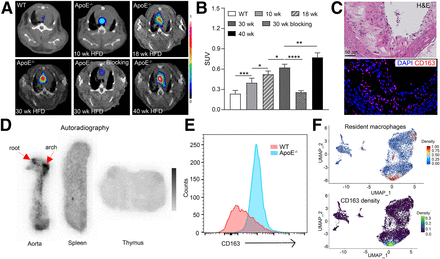
To assess the potential of [64Cu]Cu-ICT-01 for imaging atherosclerotic plaques, we performed a time course study on ApoE−/− mice from 10 to 40 wk on a high-fat diet (HFD). In contrast to the minimal radiotracer retention within the aortic arch of WT mice (SUV, 0.24 ± 0.05; n = 7), [64Cu]Cu-ICT-01 PET revealed significantly increased uptake at the aortic arch (SUV, 0.40 ± 0.07; P = 0.0001; n = 7) after 10 wk on an HFD (Figs. 2A and 2B). With the progression of atherosclerotic lesions, uptake at the aortic arch increased over time, leading to more than 2 times higher uptake (SUV, 0.77; P < 0.0001; n = 4) after 40 wk on an HFD (Figs. 2A and 2B). This was clearly illustrated in the maximum-intensity projection image (Supplemental Fig. 4A). Moreover, the intense PET signals were next to the microcalcification (a biomarker for plaques) within the aortic arch visualized by CT (Supplemental Fig. 5) (36). This coincidence between PET and CT for ApoE−/− mice after 40 wk on an HFD suggested specific binding of [64Cu]Cu-ICT-01 to plaques. Importantly, the competitive receptor blocking study performed after 30 wk on an HFD significantly (SUV, 0.26 ± 0.02; P < 0.0001; n = 4–6) reduced the radiotracer accumulation at the aortic arch, confirming the targeting specificity of [64Cu]Cu-ICT-01.
PET/CT imaging and characterization of CD163+ macrophages in ApoE−/− mice. (A) Representative PET/CT images of [64Cu]Cu-ICT-01. (B) Quantification of uptake at aortic arches in WT and ApoE−/− mice on HFD at various time points (n = 4–7/group). (C) Hematoxylin and eosin and immunofluorescence staining. (D) [64Cu]Cu-ICT-01 autoradiography of aorta, spleen, and thymus. (E) Flow cytometry. (F) Single-cell RNA sequencing of macrophages collected from ApoE−/− mouse aortas after 20 wk on HFD. Resident macrophage gene set score uses Folr2, Lyve1, and F13a1 (top) and gene expression density (bottom) for Cd163 in uniform manifold approximation and projection embedding. *P < 0.05. **P < 0.01. ***P < 0.001. ****P < 0.0001. DAPI = (4′,6-diamidino-2-phenylindole); H&E = hematoxylin and eosin; UMAP_1 = uniform manifold approximation and projection 1.
In Ldlr−/− mice after 30 wk on an HFD, [64Cu]Cu-ICT-01 revealed strong uptake (SUV, 0.58 ± 0.09; n = 6) at the aortic arch (Supplemental Fig. 6), comparable to that in ApoE−/− mice (SUV, 0.62 ± 0.05; n = 6) at the same time point, further validating the plaque-targeting efficiency of [64Cu]Cu-ICT-01.
Hematoxylin and eosin staining of the aortic arch collected from ApoE−/− mice fed an HFD for 20 wk showed significant development of atherosclerotic plaques, including an enlarged neointima, infiltration of inflammatory cells, and a large lipid pool. Immunofluorescence staining revealed extensive expression of CD163+ macrophages throughout the plaques (Fig. 2C), which was further validated by the low signals observed from control immunofluorescence staining (Supplemental Fig. 7). Quantification of immunofluorescence staining demonstrated gradually increased expression of CD163+ macrophages along with progression of atherosclerotic lesions, which showed a linear correlation with [64Cu]Cu-ICT-01 uptake at the aortic arch and plaque size, respectively, during the time course study. Because of the high expression of CD163+ macrophages on the plaques, [64Cu]Cu-ICT-01 uptake also correlated well with plaque size during the 40-wk study (Supplemental Fig. 8). Ex vivo autoradiography performed on ApoE−/− mice after 20 wk on an HFD revealed specific uptake at the aortic arch and root, with low retention observed in both spleen and thymus (Fig. 2D), consistent with the low abundance of myeloid cells among immune cells in the human spleen (37). Consistent with immunostaining data, flow cytometry demonstrated significantly higher expression of CD163+ macrophages on the aortas collected from ApoE−/− mice (53.0% ± 16.4%, n = 6) after 20 wk on an HFD than on the aortas collected from WT mice (22.3% ± 2.5%, n = 3, P < 0.05) (Fig. 2D; Supplemental Fig. 9). Moreover, single-cell RNA sequencing of aortic arteries from ApoE−/− mice after 20 wk on an HFD confirmed that CD163 is expressed with high density in a subtype of resident macrophage classified by resident macrophage–specific genes, including Folr2, Lyvel, and F13a1 (Fig. 2E; Supplemental Fig. 10) (38). These data highlighted the specific expression of Cd163 on tissue-resident macrophages in atherosclerotic lesions and the potential of CD163 as a biomarker for plaque development. The sensitive detection of CD163+ macrophages in ApoE−/− mice suggested the capability of [64Cu]Cu-ICT-01 for noninvasively tracking the progression of atherosclerotic lesions.
CD163 PET in PCSK9 Mouse Atherosclerosis Model
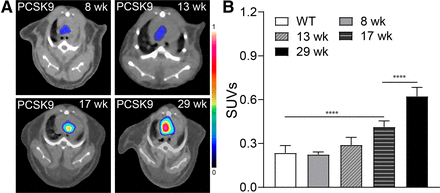
In addition to the genetically engineered ApoE−/− and Ldlr−/− murine atherosclerosis models, we assessed the plaque-targeting efficiency of [64Cu]Cu-ICT-01 using WT C57BL/6 mice treated with adenoassociated virus 2 vector encoding murine PCSK9 (39–41). Similar to WT mice, PCSK9 mice after 8 wk (SUV, 0.22 ± 0.02; n = 4) and 13 wk (SUV, 0.29 ± 0.05; n = 8) on an HFD did not show any increased radiotracer accumulation (Fig. 3), consistent with the time course of atherosclerotic lesion development in this model (39,41). After 17 wk on an HFD, [64Cu]Cu-ICT-01 showed substantially intensified radiotracer localization (SUV, 0.41 ± 0.04; P < 0.0001; n = 8) at the aortic arch, which further increased to approximately 3 times (SUV, 0.62 ± 0.06; P < 0.0001; n = 4) as much as that in WT mice after 29 wk on an HFD (Fig. 3). The strong uptake within the aortic arch was also depicted in the maximum-intensity projection image (Supplemental Fig. 4B). Moreover, consistent with the histopathologic features of plaques in ApoE−/− mice, significant development of atherosclerotic lesions and expression of CD163+ macrophages were determined in the aortic arch of PCSK9 mouse after 34 wk on an HFD (Supplemental Fig. 11), supporting the PET imaging results.
PET/CT imaging and characterization of CD163+ macrophages in PCSK9 mice. (A) Representative PET/CT images of [64Cu]Cu-ICT-01 in PCSK9 mice. (B) Quantification of uptake at aortic arches at various time points on HFD (n = 4–8/group). ****P < 0.0001.
Assessment of CD163 Expression on Human Atherosclerotic Specimens
To assess the translational potential of CD163 as a biomarker for human atherosclerosis, we characterized human plaque specimens collected from carotid endarterectomy (CEA) to assess their CD163 expression levels and related histopathologic features. Hematoxylin and eosin staining of the maximally diseased portion of CEA specimens showed a large fibrous capsule, a necrotic core, significant calcification, substantial intraplaque hemorrhage, and high infiltration of inflammatory cells. Immunofluorescence staining showed high expression of CD163+ macrophages across the tissue, with most signal associated with the infiltrated inflammatory cells and intraplaque hemorrhage but not the fibrous capsule (Fig. 4A). Characterization of additional CEA tissues demonstrated high expression of CD163+ macrophages on both maximally and minimally diseased portions. Particularly, intense accumulation of CD163+ macrophages was observed at the shoulder regions of these CEA specimens independently of the difference in histopathologic features (Supplemental Fig. 12). We next assessed the potential of [64Cu]Cu-ICT-01 binding to human CD163 via ex vivo autoradiography following our established protocol (42). As shown in Figure 4B, [64Cu]Cu-ICT-01 revealed intensive and heterogeneous binding to the CEA specimen, and this binding could be effectively blocked using nonradioactive ICT-01, indicating binding specificity. Moreover, both region-of-interest–based and whole-tissue analyses of the coregistered images demonstrated a linear correlation between [64Cu]Cu-ICT-01 autoradiography and CD163 immunostaining, further confirming the binding specificity of [64Cu]Cu-ICT-01 to human CD163.
Ex vivo characterization of human CEA specimen. (A) Hematoxylin and eosin staining showed advanced plaque with large fibrous core, intraplaque hemorrhage (asterisk, green box and magnified image), calcification, and infiltration of inflammatory cells. CD163 immunofluorescence staining showed extensive expression of CD163 across tissue, with most signals associated with intraplaque hemorrhage (green box and magnified image) and inflammatory cells. (B) [64Cu]Cu-ICT-01 autoradiography showed high binding to CD163 in CEA tissue. Blocking revealed significantly decreased signals, suggesting binding specificity. Both region-of-interest and whole-tissue analyses on coregistered autoradiography and immunofluorescence staining showed good correlation. DAPI = (4′,6-diamidino-2-phenylindole).
DISCUSSION
The complex pathophysiology of atherosclerosis reflects a systemic proinflammatory process involving innate and adaptive immune responses engaging multiple immune cells such as monocytes, macrophages, and T cells. The multiplex interplay among these processes is characterized by chronicity but with dynamic variation that yields plaque lesions with constantly evolving biologic and anatomic features that determine symptomatology and the induction of clinical events (1,3). Most importantly, the complicated pathogenesis of atherosclerosis precludes clinically available anatomic imaging modalities from reliably visualizing specific cellular features or biologic processes that identify high-risk individuals for immunomodulatory or targeted treatment (17,43).
Of the various immune cells, macrophages are pivotal in the chronic inflammatory processes that drive the pathogenesis of atherosclerosis, making them the most studied immune cells for atherosclerosis diagnosis (4,6,7). It is known that macrophages are hallmarked by phenotypic heterogeneity and express a spectrum of activational programs that exist as a function of their immediate surroundings, which highlights the importance of imaging specific subtypes of macrophages. Previously, we reported targeted PET of monocyte-derived proinflammatory chemokine receptor 2–positive macrophages that are instrumental to the plaques’ initiation, progression, and complication (18,20,44). Moreover, the effects of antiinflammatory tissue-resident macrophages that are thought to counterbalance proinflammatory macrophages by promoting the resolution of inflammation and tissue repair on atherosclerotic plaque development are insufficiently studied. Although some radiotracers targeting resident macrophages have been reported, their imaging efficiency and targeting specificities to track the dynamic variation in this subtype of macrophages along with plaque progression and complication, especially in humans, needs further validation (7,21,22).
Because of the specific expression of CD163 on a subtype of tissue-resident macrophages and their exclusive function to clear hemoglobin–haptoglobin complexes after intraplaque hemorrhage, the presence of CD163+ macrophages is closely associated with a vulnerable phenotype of human carotid plaques (26,28,30,45), making CD163 a unique target to assess plaque vulnerability in contrast to other macrophage-targeted biomarkers (4,7). Therefore, we screened a 12-mer peptide binding to CD163 and developed a CD163-targeted PET radiotracer. In contrast to other PET radiotracers used for cardiovascular disease imaging, peptide-based radiotracers have unique advantages including rapid blood and renal clearance and low nonspecific retention in major organs, which may improve the target-to-background contrast ratio. In vitro and in vivo studies demonstrated the stability of [64Cu]Cu-ICT-01. Cell-binding assays revealed high and comparable binding affinities to both mouse and human CD163+ cells despite the fact that ICT-01 was screened from human protein. Biodistribution studies of [64Cu]Cu-ICT-01 showed rapid clearance and low blood retention, which may enable the detection of small atherosclerotic lesions during plaque initiation and sensitive tracking of plaque progression. The significantly decreased uptake in immune organs such as liver, spleen, and thymus after competitive blockade demonstrated the presence of CD163+ resident macrophages and suggested the targeting specificity of [64Cu]Cu-ICT-01. Moreover, among various positron emitters for NODAGA radiolabeling, the relatively short positron range of 64Cu makes it favorable for cardiovascular applications (46). Additionally, its 12.7-h decay half-life enables shipping of 64Cu-radiolabeled radiotracers to a second site for preclinical or clinical studies.
The plaque-targeting efficiency of [64Cu]Cu-ICT-01 was demonstrated in multiple mouse atherosclerosis models using PET/CT and was further confirmed by ex vivo autoradiography. The competitive receptor blocking confirmed the specificity with which [64Cu]Cu-ICT-01 detects CD163+ macrophages on plaques. Moreover, the high binding affinity of [64Cu]Cu-ICT-01 and favorable pharmacokinetics enabled sensitive detection of the subtle changes in CD163+ macrophage populations and plaque development in both ApoE−/− and PCSK9 mice along with progression of atherosclerosis. This sensitivity was further demonstrated by the linear correlation between [64Cu]Cu-ICT-01 uptake at the aortic arch and CD163 expression, as well as the plaque sizes during the longitudinal studies. These results highlighted the potential of [64Cu]Cu-ICT-01 as a diagnosis tool to facilitate the development of CD163-targeted treatment (47).
Immunostaining showed increased expression of CD163+ macrophages along with progression of atherosclerosis and correlation with plaque sizes, indicating the importance of CD163+ macrophages in plaque development. Moreover, flow cytometry and single-cell RNA sequencing data confirmed the specific expression of CD163 on the tissue-resident macrophages. The distinct expression pattern from monocyte-derived macrophages highlighted the significance of CD163+ macrophage atherosclerosis pathogenesis. Importantly, the high sensitivity and specificity of [64Cu]Cu-ICT-01 in detecting CD163+ macrophages may be able to track the progression of atherosclerotic lesions, underscoring the potential of this radiotracer for further investigation.
We demonstrated elevated expression of CD163 at atherosclerotic lesions in deidentified human CEA specimens. The high accumulation of CD163+ macrophages in these plaques at the shoulder regions and associated intraplaque hemorrhage were consistent with previous reports (26,45), suggesting a correlation between CD163 expression and plaque vulnerability. Moreover, ex vivo characterization of human CEA specimens demonstrated higher expression of CD163+ macrophages in symptomatic patients than in asymptomatic patients (45), highlighting the potential of CD163 as a biomarker to assess plaque vulnerability.
Our study had several limitations. All the mouse atherosclerosis models that we used require an HFD that does not reflect the humanlike lipid profile. Although severe atherosclerotic lesions were observed in these models, none of the plaques ruptured. Future studies are needed on rupture-prone animal models to assess the potential of [64Cu]Cu-ICT-01 for detecting plaque vulnerability (48). Another limitation is that only a limited number of human CEA specimens were used to assess expression of CD163+ macrophages. A large cohort of retrospective CEA tissues integrating patient demographic information and symptoms is warranted to thoroughly evaluate the potential of CD163 as a biomarker for vulnerable plaques and of [64Cu]Cu-ICT-01 PET/CT as a way to assess the rupture index in atherosclerotic patients.
CONCLUSION
On the basis of phage display screening, we developed a CD163-targeting radiotracer, [64Cu]Cu-ICT-01, with high binding affinity and a favorable biodistribution profile. In vivo PET/CT imaging in mouse atherosclerosis models revealed the sensitivity and specificity of [64Cu]Cu-ICT-01 in targeting plaques. Molecular characterization of mouse aortas demonstrated the high and unique expression of CD163 on tissue-resident macrophages. Ex vivo human CEA tissue characterization demonstrated the potential of CD163 as a biomarker to assess plaque rupture index and warrants further studies in future translational settings to evaluate [64Cu]Cu-ICT-01 PET/CT for risk stratification of atherosclerotic patients.
DISCLOSURE
The preclinical imaging facility for animal PET/CT scans is supported by NIH/NCI Siteman Cancer Center support grant P30CA091842, NIH instrumentation grants S10OD018515 and S10OD030403, and internal funds from Mallinckrodt Institute of Radiology. The human imaging was performed at the Center for Clinical Imaging Research, which is supported by Mallinckrodt Institute of Radiology. Benjamin Kopecky was supported by a grant from the NIH (K08HL159359). Kory Lavine is supported by grants from the NIH (R35HL161185) and the Leducq Foundation (20CVD02). Yongjian Liu is supported by grants from the NIH (R01HL153436, R01HL150891, R01HL151685, P41EB025815, R35 HL145212) and the Leducq Foundation (20CVD02). No other potential conflict of interest relevant to this article was reported.
KEY POINTS
QUESTION: Can [64Cu]Cu-ICT-01 be used to specifically image tissue-resident macrophages with PET?
PERTINENT FINDINGS: [64Cu]Cu-ICT-01 showed high binding affinity to CD163 and a favorable biodistribution profile. In vivo imaging in mouse atherosclerosis models demonstrated sensitive and specific detection of CD163+ resident macrophages upregulated on plaques. The unique expression of CD163 on resident macrophages was further confirmed by ex vivo tissue characterization. Elevated expression of CD163 associated with intraplaque hemorrhage on human carotid plaques was confirmed.
IMPLICATIONS FOR PATIENT CARE: CD163 expression is associated with vulnerability of human carotid plaques. Targeted imaging using [64Cu]Cu-ICT-01 PET/CT may provide new insights into the role of CD163+ macrophages in the pathogenesis of atherosclerosis and be a useful tool to risk-stratify patients for appropriate management.
Footnotes
Published online Mar. 28, 2024.
- © 2024 by the Society of Nuclear Medicine and Molecular Imaging.
REFERENCES
- Received for publication October 23, 2023.
- Revision received February 26, 2024.