Visual Abstract
Abstract
This single-center, single-arm, phase II trial (ChiCTR2100050057) investigated the ability of 18F-labeled fibroblast activation protein inhibitor ([18F]AlF-NOTA-FAPI-04, denoted as 18F-FAPI) PET/CT to predict the response to neoadjuvant camrelizumab plus chemotherapy (nCC) in locally advanced esophageal squamous cell carcinoma (LA-ESCC). Methods: This study included 32 newly diagnosed LA-ESCC participants who underwent 18F-FAPI PET/CT at baseline, of whom 23 also underwent scanning after 2 cycles of nCC. The participants underwent surgery after 2 cycles of nCC. Recorded PET parameters included maximum, peak, and mean SUVs and tumor-to-background ratios (TBRs), metabolic tumor volume, and total lesion FAP expression. PET parameters were compared between patient groups with good and poor pathologic responses, and the predictive performance for treatment response was analyzed. Results: The good and poor response groups each included 16 participants (16/32, 50.0%). On 18F-FAPI PET/CT, the posttreatment SUVs were significantly lower in good responders than in poor responders, whereas the changes in SUVs with treatment were significantly higher (all P < 0.05). SUVmax (area under the curve [AUC], 0.87; P = 0.0026), SUVpeak (AUC, 0.89; P = 0.0017), SUVmean (AUC, 0.88; P = 0.0021), TBRmax (AUC, 0.86; P = 0.0031), and TBRmean (AUC, 0.88; P = 0.0021) after nCC were significant predictors of pathologic response to nCC, with sensitivities of 63.64%–81.82% and specificities of 83.33%–100%. Changes in SUVmax (AUC, 0.81; P = 0.0116), SUVpeak (AUC, 0.82; P = 0.0097), SUVmean (AUC, 0.81; P = 0.0116), and TBRmean (AUC, 0.74; P = 0.0489) also were significant predictors of the pathologic response to nCC, with sensitivities and specificities in similar ranges. Conclusion: 18F-FAPI PET/CT parameters after treatment and their changes from baseline can predict the pathologic response to nCC in LA-ESCC participants.
The results of the CROSS trial and the 5010 trial laid the foundation for concurrent chemoradiotherapy as a standard neoadjuvant therapy for locally advanced esophageal squamous cell carcinoma (LA-ESCC), with pathologic complete response (pCR) rates of 49% and 43.3% reported by the respective studies (1–3). However, 40%–50% of LA-ESCC patients still experience tumor recurrence after neoadjuvant concurrent chemoradiotherapy, and distant metastasis accounts for about 71%–80.5% of all cases of relapse. Although neoadjuvant chemoradiotherapy can improve the pathologic response rate among LA-ESCC patients, the incidence of postoperative complications is high, and whether this treatment strategy improves long-term survival remains controversial. Therefore, researchers continue to actively explore better systemic treatments for LA-ESCC.
Several clinical trials have published data on the efficacy of immunotherapy as second-line treatment for esophageal cancer, and the results have confirmed the benefit for patients (4–6). Therefore, in recent years, researchers have explored immunotherapy plus chemotherapy as a neoadjuvant therapy for LA-ESCC. The NICE trial, the KEEP-G03 trial, and the toripalimab trail showed that neoadjuvant immunotherapy plus chemotherapy can lead to pCR rates of 16.67%–45.5% and lower the incidence of postoperative complications compared with neoadjuvant chemoradiotherapy in patients with esophageal cancer (7–9), and the overall treatment effectiveness apparently varies between them. Dziadek et al. reported that esophageal cancer highly expresses fibroblast-activating protein (FAP), and thus, high FAP inhibitor (FAPI) uptake is common at baseline (10). FAP might be a cancer immunotherapy biomarker with a multifaceted role in therapy response. Therefore, it is necessary to monitor changes after treatment. Effective methods for predicting the response to immunotherapy plus chemotherapy are urgently needed for accurate screening of esophageal cancer patients to identify those most likely to benefit from the combined treatment.
Tumor stromal cells are a core feature of the tumor microenvironment, and the efficacy of immunotherapy is closely related to characteristics of both the tumor and the tumor microenvironment (11). FAP can promote tumor cell proliferation, migration, and invasion, resulting in drug resistance and immunosuppression (12,13). Accordingly, FAP is a potential biomarker for predicting the efficacy of tumor therapy. Our previous studies have shown that [18F]AlF-NOTA-FAPI-04 (denoted as 18F-FAPI) for PET offers high specificity as a tracer for FAP imaging and allows fast imaging with high contrast in LA-ESCC, lung cancer, and other various cancers (14), thereby enabling the systematic investigation of the ability of 18F-FAPI PET parameters to predict treatment efficacy. Our research has confirmed that 18F-FAPI PET parameters at baseline can predict the short-term efficacy of concurrent chemoradiotherapy (15). However, the predictive ability of 18F-FAPI PET for the efficacy of neoadjuvant immunotherapy plus chemotherapy in LA-ESCC remains unclear.
The present study was a single-center, single-arm, phase II trial evaluating the predictive value of 18F-FAPI PET/CT for the response to neoadjuvant camrelizumab plus chemotherapy (nCC) in participants with LA-ESCC.
MATERIALS AND METHODS
Participants
This is an interim report of a single-center, single-arm phase II study evaluating the predictive value of [18F]AlF-NOTA-FAPI-04 (denoted as 18F-FAPI) PET/CT for the response of LA-ESCC to camrelizumab plus chemotherapy. The study was approved by the Clinical Research Ethics Committee of our institution (ChiCTR2100050057). The institutional review board approved this study, and all subjects gave written informed consent.
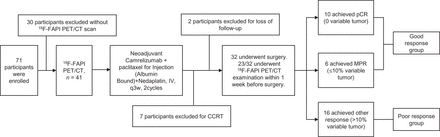
A flowchart of the study design and the detailed inclusion and exclusion criteria are presented in Figure 1 and the supplemental materials (supplemental materials are available at http://jnm.snmjournals.org).
Study flowchart. CCRT = concurrent chemoradiotherapy; MPR = major pathologic response.
Treatment
The treatment plan consisted of preoperative administration of anti–PD-1 antibody, camrelizumab, and chemotherapy followed by surgery. All participants were scheduled to receive 2 cycles of neoadjuvant therapy consisting of a fixed dose of 200 mg of camrelizumab every 3 wk as well as chemotherapy. The selected dose of Nab-paclitaxel administered in this study was 260 mg/m2 (day 1), and that of nedaplatin was 80–100 mg/m2 (day 1), with a 3-wk administration cycle. Treatment was interrupted or delayed if a severe adverse event occurred. Before and approximately 4–6 wk after neoadjuvant treatment, each participant was evaluated by CT of the chest and upper abdomen and 18F-FAPI PET/CT.
18F-FAPI PET/CT Scanning
18F-FAPI was synthesized as described previously (16). Participants were not required to fast before scanning, nor was blood glucose measurement required. After intravenous injection of 18F-FAPI (225 ± 28 MBq), the participant rested for approximately 1 h. Scanning was then performed with an integrated in-line PET/CT system (Gemini TF Big Bore; Philips Healthcare). Whole-body CT scans were obtained using a low-dose protocol (300 mAs, 120 kV, 512 × 512 matrix, rotation time of 1.0 s, and pitch index of 0.688; reconstructed with a soft-tissue kernel to a slice thickness of 2 mm) for attenuation correction. PET data were acquired in 3-dimensional mode using a 200 × 200 matrix with an imaging time of 1 min per bed position. During image acquisition, the participant maintained normal shallow breathing. Subsequently, after attenuation and correction (Body-ctac-SB. Lstcln [Biograph 3-dimensional iterative reconstruction software], with time-of-flight correction), the attenuation-corrected PET images, CT images, and fused PET/CT images were generated for clinical reading and analysis.
Imaging Analysis
The attenuation-corrected PET images, CT images, and fused PET/CT images, displayed as coronal, sagittal, and transaxial slices, were viewed and analyzed on a nuclear medicine information system (Beijing Mozi Healthcare Ltd.). Two experienced nuclear medicine physicians visually assessed the 18F-FAPI PET/CT images and reached consensus regarding the image interpretations for primary and metastatic tumors.
Positive criteria were an obvious concentration of 18F-FAPI, a significant reduction in tumor size after nCC, a significant tumor size enlargement after nCC, no significant change in tumor diameter but a significant decrease in 18F-FAPI uptake after nCC, and the presence of metastatic disease on clinical examination and routine imaging. Negative criteria were obvious uptake of 18F-FAPI PET/CT in benign lymph nodes, or lymph nodes with a short diameter of more than 1 cm without uptake and negative findings on clinical examination and routine imaging.
On transaxial sections, regions of interest were drawn around tumor lesions with higher uptake, and 18F-FAPI PET/CT parameters were generated by an automated 3-dimensional contouring program with a 30% isocontour. Each region of interest was normalized to the injected dose per kilogram of the participants’ body weight to determine the SUVs, which were calculated according to the following formula: [measured activity concentration (Bq/mL) × body weight (g)]/injected activity (Bq). The obtained parameters, including the SUVmax, SUVmean, SUVpeak, metabolic tumor volume (MTV), and total lesion FAP expression (TLF), were determined by an automated contouring program provided by Beijing Mozi Healthcare Ltd. We also measured the SUVmean in 1 cm3 areas in the pulmonary aortic trunk. Then, the ratios of SUVmax and SUVmean for the primary tumor to the SUVmean of the pulmonary artery were calculated and denoted as the maximum and mean tumor-to-background ratios (TBRmax and TBRmean, respectively). If disagreement occurred, discussion among the imaging experts with consideration of the results from other imaging modalities proceeded until a final consensus was reached. The changes in uptake values from before to after nCC are denoted as ΔSUVmax, ΔSUVmean, ΔSUVpeak, ΔTBRmax, and ΔTBRmean.
Response Evaluation and Pathologic Assessment
The primary endpoint was pathologic response, which was assessed by the tumor regression grade (TRG) determined using the Chirieac system (17). Secondary endpoints included the proportion of patients with complete surgical resection and the pCR rate, defined as the proportion of patients who experienced a pCR. Surgical outcome was described as the R0 resection rate (defined as the rate of negative margins microscopically). Outcomes of pCR or major pathologic response (tumor regression > 90%) were classified as good responses, and outcomes of stable disease or other disease were classified as poor responses.
Postoperative LA-ESCC specimens were examined histopathologically, and the TRG was scored as follows: grade 0, complete regression with no viable cancer cells; grade 1, moderate regression with single cancer cells or a small cluster of cancer cells; grade 2, minimal regression with residual cancer but less than fibrosis; or grade 3, poor regression with extensive residual cancer, or minimal or no cancer cell death (17).
Statistical Analysis
Statistical analysis was performed using IBM SPSS Statistics, version 25.0, for Microsoft Windows. Normally distributed data are expressed as mean ± SD, and nonnormally distributed data are expressed as median with interquartile range. The Mann–Whitney U test was used to compare the 18F-FAPI PET/CT parameters between the good and poor response groups. Logistic regression analyses were performed to identify 18F-FAPI PET/CT parameters related to the TRG. Receiver operating characteristic curve analysis was conducted to determine the threshold values with the maximum Youden index as well as the predictive accuracy of 18F-FAPI PET/CT parameters for pathologic response of LA-ESCC to nCC. All tests were 2-sided, and values of P less than 0.05 defined statistical significance.
RESULTS
Patient Characteristics, Treatments, and Outcomes
Between August 13, 2021, and December 31, 2022, 71 participants newly diagnosed with LA-ESCC were screened, and after application of the inclusion and exclusion criteria for the study, 32 were enrolled. The baseline characteristics of the enrolled participants are presented in Table 1. All participants received neoadjuvant treatment with 2 cycles of camrelizumab plus Nab-paclitaxel and nedaplatin. The median interval between the last administration of neoadjuvant therapy and surgery was 36.5 d (range, 32–41 d).
Clinical and Pathologic Characteristics of Patients
As surgical treatment, 29 participants (90.6%) underwent video-assisted thoracoscopic surgery, and 3 participants (9.4%) underwent thoracotomy. R0 surgical resection was achieved in 30 patients (93.8%), and R1 or R2 surgical resection was achieved in 2 participants (6.2%). Delay of surgical treatment due to adverse events occurred in 2 of the 32 participants (6.2%).
The pCR rate among the enrolled LA-ESCC participants was 31% (10/32), and a major pathologic response was achieved in 50% of participants (16/32, including the pCR patients).
Comparison of SUVs at Baseline and After nCC
The SUVs at baseline and after nCC are listed in Table 2. Compared with baseline values, significantly decreases in SUVmax, SUVpeak, SUVmean, TBRmax, and TBRmean were observed after nCC (all P < 0.005). MTV showed no change from baseline (30.66 ± 20.95) to after nCC (40.99 ± 32.55, P = 0.19).
Comparison of 18F-FAPI PET/CT Parameters at Baseline and After Neoadjuvant Therapy
Comparison of SUVs According to Response to nCC
The results in Table 3 and Figure 2 illustrate the differences in SUVs between the participant groups with a good response to treatment and a poor response to treatment. At baseline, no differences in SUVmax, SUVpeak, SUVmean, TBRmax, TBRmean, TLF, or MTV were observed between the groups (all P > 0.05). After nCC, the SUVmax, SUVpeak, SUVmean, TBRmax, and TBRmean were lower in the good response group than in the poor response group (all P < 0.05).
Comparison of 18F-FAPI PET/CT Parameters at Different Time Points Between Patient Groups with Good and Poor Responses to nCC
(A–E) Representative 18F-FAPI PET/CT scans for 5 study participants who experienced good response to treatment. Images show that tracer uptake is significantly decreased or close to background level after nCC. (F–J) Representative 18F-FAPI PET/CT scans for 5 study participants who experienced poor response to treatment. Images show slight decreases in tracer uptake after neoadjuvant therapy. Arrows indicate foci.
The changes in 18F-FAPI PET/CT parameters from baseline to after nCC also were also analyzed and compared between the good and poor response groups (Table 3). The ΔSUVmax, ΔSUVpeak, ΔSUVmean, ΔTBRmax, and ΔTBRmean were significantly higher in the good response group than in the poor response group (all P < 0.05).
Correlations Between SUVs and TRG
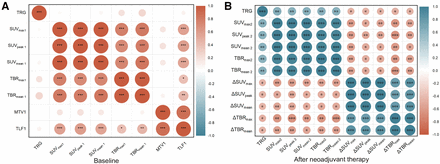
Figure 3 presents the results for correlations identified between 18F-FAPI PET/CT parameters and the pathologic features of LA-ESCC. First, no significant correlation was observed between TRG and programmed death ligand 1 (PD-L1) status in LA-ESCC or between TRG and the SUVs at baseline. However, significant positive correlations were found between the SUVmax, SUVpeak, SUVmean, TBRmax, and TBRmean after nCC and TRG (P = 0.0018, P < 0.001, P = 0.0023, P = 0.0020, and P = 0.0001, respectively). No correlation was found between TLF or MTV and TRG (P = 0.4469 and P = 0.0708, respectively).
Correlations between 18F-FAPI PET/CT parameters and TRG (Spearman coefficient) at baseline and after treatment. Red represents positive correlation between 2 variables, and blue represents negative correlation. Stronger correlations are in darker colors. *P < 0.05. **P < 0.01. ***P < 0.001.
For the changes in 18F-FAPI PET/CT parameters from baseline to after nCC, significantly negative correlations were found between ΔSUVmax, ΔTBRmax, ΔSUVpeak, ΔSUVmean, and ΔTBRmean and TRG (P = 0.0273, P = 0.0125, P = 0.0350, P = 0.0359, and P = 0.0172, respectively).
Predictive Performance of SUVs for Pathologic Response of LA-ESCC to nCC
Receiver operating characteristic curve analysis was performed to assess the accuracy of 18F-FAPI PET/CT parameters for predicting good and poor pathologic responses to nCC in patients with LA-ESCC (Fig. 4; Table 4). The following parameters measured after nCC were found to be significant predictors of pathologic response to nCC: SUVmax (area under the curve [AUC], 0.87; P = 0.0026; sensitivity, 63.64%; and specificity, 100%), SUVpeak (AUC, 0.89; P = 0.0017; sensitivity, 81.82%; and specificity, 83.33%,), SUVmean (AUC, 0.88; P = 0.0021; sensitivity, 81.82%; and specificity, 83.33%), TBRmax (AUC, 0.86; P = 0.0031; sensitivity, 81.82%; and specificity, 91.67%), and TBRmean (AUC, 0.88; P = 0.0021; sensitivity, 81.82%; and specificity, 91.67%). Additionally, the pre- to posttreatment changes in the following 18F-FAPI PET/CT parameters also were identified as significant predictors of pathologic response to nCC: ΔSUVmax (AUC, 0.81; P = 0.0116; sensitivity, 81.82%; and specificity, 83.33%), ΔSUVpeak (AUC, 0.82; P = 0.0097; sensitivity, 72.73%; and specificity, 83.33%), ΔSUVmean (AUC, 0.81; P = 0.0116; sensitivity, 81.82%; and specificity, 75.0%), and ΔTBRmean (AUC, 0.74; P = 0.0489; sensitivity, 63.64%; and specificity, 91.67%).
Receiver operating characteristic curves for assessing predictive accuracy of SUVs on 18F-FAPI PET/CT for identifying good and poor pathologic responders to nCC among participants with LA-ESCC. SUVmax2 = SUVmax after nCC; SUVpeak2 = SUVpeak after nCC; SUVmean2 = SUVmean after nCC; TBRmax2 = ratio of SUVmax to SUVmean of blood after nCC; TBRmean2 = ratio of SUVmean to SUVmean of blood after nCC.
Performance of PET Parameters in Prediction of Pathologic Response to nCC
DISCUSSION
As a novel imaging technique, 18F-FAPI PET/CT has been a focus of research in recent years, and its value in the diagnosis and treatment of solid tumors has been widely recognized. In the secondary analysis of this registered phase II clinical study, we preliminarily confirmed the predictive value of 18F-FAPI PET/CT for the pathologic response of LA-ESCC to nCC. In this study, participants with lower 18F-FAPI SUVs on PET/CT after therapy and larger changes in SUVs from before to after treatment were more likely to experience good responses to nCC.
Compared with the baseline values for 18F-FAPI PET/CT parameters, all SUVs decreased significantly after nCC. Wahl et al. published PERCIST 1.0 (18), which require a 30% decline in SUV for a response. Zhao et al. reported a case supporting the use of [68Ga]Ga-DOTA-FAPI-04 imaging to monitor early response to chemoradiotherapy in a participant with esophageal cancer, based on greatly reduced [68Ga]Ga-DOTA-FAPI-04 uptake in the primary lesion (19). Relevant studies of esophageal cancer with larger sample sizes have not been reported, but Miao et al. also found that 68Ga-FAPI-04 imaging could be used for early prediction of pathologic response to neoadjuvant chemotherapy in locally advanced gastric cancer, based on significant decreases in SUVmax, SUVpeak, and TBR after 1 cycle of neoadjuvant chemotherapy (20). Therefore, the changes in SUVs after treatment represent important markers of whether antitumor therapy has been effective.
Hu et al. reported that baseline 18F-FAPI PET uptake can predict the short-term efficacy of concurrent chemoradiotherapy (15). In this study, we found that baseline uptake of 18F-FAPI PET did not predict the pathologic response rate to chemotherapy combined with immunotherapy. The most important difference is that the treatment methods for the 2 cohorts were different, and the ultimate purposes of the studies were different. Although Hu et al. mainly analyzed the objective response rate, the present study mainly analyzed the pathologic response rate. In addition, esophagitis is a common complication in patients who undergo thoracic radiation therapy and a source of considerable morbidity (21). As the case reported by Yang et al. shows, intense 18F-FAPI activity was noted in esophagitis (22). This is one of the main reasons why Hu et al. did not analyze the change value in the 2 sets of PET data.
In the present study, SUVs and ΔSUVs on 18F-FAPI PET/CT significantly correlated with TRG and were identified as significant predictors of the response to nCC in participants with LA-ESCC, whereas TLF and MTV were not. The application of 18F-FAPI PET for predicting tumor response to antitumor therapy has been explored previously (23–25), but this is the first study, to our knowledge, to explore its predictive value for response to nCC. Miao et al. also found that changes in SUVmax and TBR were good predictors of neoadjuvant chemotherapy in patients with gastric cancer (20), and Zhu et al. reported that changes in SUVmax, TFL, and MTV are associated with good treatment response in participants with inoperable pancreatic ductal adenocarcinoma (24). Consistent with these previous studies, although we studied different PET parameters in a different type of cancer, we observed that similar changes in SUVs could predict well the curative effect of cancer treatment. These findings indicate that for tumors with smaller decreases in SUVs, a higher dose than prescribed by current protocols should be delivered, or another chemotherapy regimen should be administered to achieve better tumor control.
What are the advantages of 18F-FAPI over 18F-FDG PET for neoadjuvant chemotherapy combined with immunotherapy in esophageal cancer? First, according to imaging theory,18F-FDG PET is mainly for glucose metabolism imaging, whereas 18F-FAPI PET is mainly for FAP imaging. 18F-FDG PET requires patients to fast before examination, and the blood glucose level is strictly limited, which leads to the 18F-FDG PET examination’s not being suitable for some diabetic patients. However, 18F-FAPI PET imaging does not require a specific patient blood glucose level and does not require the patient to fast, making it simpler and easier. Second, there have been no reports on the use of 18F-FDG PET/CT to monitor the response to neoadjuvant immunotherapy for esophageal cancer, which may be due to the fact that neoadjuvant immunotherapy for esophageal cancer is a newly emerging research hot spot in recent years. A systematic review of 13 studies, largely of retrospective cohorts, showed modest evidence, with 8 studies showing a correlation between 18F-FDG PET parameters and pathologic response or the studied clinical outcome after neoadjuvant chemoradiotherapy (26). One study of 31 patients with resectable LA-ESCC or adenocarcinoma were followed prospectively with 18F-FDG PET during treatment with trimodality therapy, and the results showed that baseline total lesion glycolysis and postchemoradiotherapy total lesion glycolysis were associated with overall survival (27). In another study of esophageal cancer patients, a decrease in glucose SUV of 35% or greater was defined as response and associated with a median overall survival that was better than that of nonresponders, with a hazard ratio of 2.13 (95% CI, 1.14–3.99; P = 0.015) (28). It is worth noting that Chen et al.’s study confirmed the predictive value of 18F-FDG PET/CT for the pathologic remission rate after neoadjuvant immunotherapy in lung cancer (29). Third, Dziadek et al. reported that high FAP expression was found in esophageal primary cancer and metastatic disease samples (10). In murine models, the pivotal role of FAP-positive cancer-associated fibroblasts in promoting tumor growth by suppressing antitumor immunity has been established (30). Therefore, FAP might be a potential biomarker for cancer immunotherapy. Backhaus et al. reported that 18F-FAPI PET/MRI could be used to assess the response of breast cancer to neoadjuvant chemotherapy, with a mean breast TBR for pCR of 0.9 (range, 0.6–1.2) and for no pCR of 2.1 (range, 1.4–3.1) (P = 0.001) (31).
Currently, PD-L1 expression and tumor mutational burden are commonly used for clinical prediction of the efficacy of immunotherapy. These factors represent molecular characteristics of tumor cells and require point sampling to obtain samples for testing. Accordingly, these predictive markers have some limitations, such as trauma due to point sampling instead of surface sampling, sampling error, and poor repeatability. Notably, the 18F-FAPI PET/CT scanning parameters investigated in the present study offer a noninvasive approach to predicting the efficacy of nCC in LA-ESCC participants. Additionally, in contrast to the SUVs and changes in SUVs on 18F-FAPI PET/CT after nCC, which significantly correlated with TRG in participants with LA-ESCC, PD-L1 status showed no correlation with TRG in the present study. Consistently, a previous metaanalysis found that a weak or no association was observed between PD-L1 expression level and survival in esophageal cancer (32). However, Ji et al. reported a significantly higher disease control rate in immune checkpoint inhibitor–treated LA-ESCC participants, as well as better survival rates for participants with high soluble PD-L1 levels (33). This may be due to the fact that PD-L1 status is determined before treatment in samples obtained by point puncture and its expression is specifically analyzed on the surface of tumor cells. In contrast, the SUVs on 18F-FAPI PET reflect the characteristics both of the tumor stroma and of the tumor cells, and PET images can monitor changes in the state of 18F-FAPI binding stereoscopically, comprehensively, and dynamically, providing better accuracy and repeatability than PD-L1 detection.
Limitations of this study include the small sample size from a single center. Additionally, the results of biomarker analysis are exploratory, obtained from a limited number of pre- and posttreatment samples, and thus, additional studies are needed to provide more precise and in-depth molecular characterization of the correlations between the 18F-FAPI PET/CT parameters and the tumor response to treatment.
CONCLUSION
Consistent with the good diagnostic value demonstrated for 18F-FAPI PET/CT in a variety of solid tumors, this study demonstrated the potential predictive value of 18F-FAPI PET/CT for the response to nCC in participants with LA-ESCC. More clinical research on this new technique for functional imaging of the tumor microenvironment is warranted and can be used to guide screening for patient populations most likely to benefit from immunotherapy.
DISCLOSURE
Yuchun Wei has received grants from the Natural Science Foundation of Shandong Province (ZR2021QH008), the China Postdoctoral Science Foundation (2023M731484), and the National Natural Science Foundation of China (82203218). No other potential conflict of interest relevant to this article was reported.
KEY POINTS
QUESTION: Can an 18F-FAPI PET/CT scan predict pathologic response to nCC in participants with resectable LA-ESCC?
PERTINENT FINDINGS: Participants with lower 18F-FAPI SUVs on PET/CT after nCC and larger changes in SUVs from before to after treatment were more likely to experience a good response to nCC.
IMPLICATIONS FOR PATIENT CARE: 18F-FAPI PET/CT scanning can be used to guide screening for patient populations most likely to benefit from neoadjuvant immunotherapy and chemotherapy.
ACKNOWLEDGMENTS
We are grateful to all patients, their families, and the site investigators who participated in the study. Additionally, we thank Jiangsu Hengrui Pharmaceuticals Co., Ltd., for supplying camrelizumab for the study and Dr. Yanyan Lin (employed by Jiangsu Hengrui Pharmaceuticals) for the data collection. We also thank Laney Weber, PhD, ELS, for English language editing services.
Footnotes
Published online Sep. 26, 2024.
- © 2024 by the Society of Nuclear Medicine and Molecular Imaging.
REFERENCES
- Received for publication August 6, 2024.
- Accepted for publication September 6, 2024.