Abstract
P28
Introduction: mRNA technology has opened new avenues to enhance the efficacy of cancer immunotherapy. However, inefficient naked mRNA delivery and lack of specific local in-vivo delivery tracking profile by non-specific carriers has remained as major hurdles to achieve optimal anti-tumor therapeutic efficacy. In this study, we hypothesized that a new carrier, cationic hyper-branched cyclodextrin-based polymers (Ppoly), can improve mRNA delivery in melanoma and used the fluorescence imaging of EGFP-mRNA to guide the mRNA delivery in-vivo and in-vitro to develop a theranostic approach.
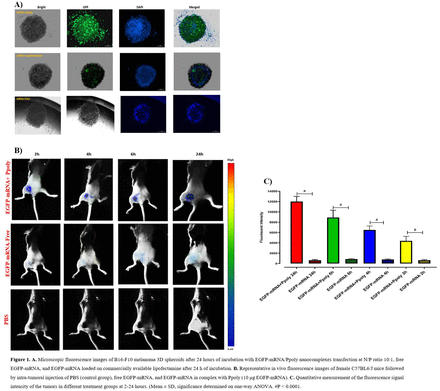
Methods: The B16-F10 mouse melanoma cell lines were used. The transfection efficiency of encoding green fluorescent protein (EGFP) mRNA loaded on Ppoly was compared to free EGFP-mRNA and Lipofectamine, a commonly used lipopolyamine formulation specifically designed as a gene delivery carrier to achieve high transfection efficiency, in both 2- and 3-dimentional (2D and 3D) cell cultures. The ability of Ppoly to deliver EGFP-mRNA inside the melanoma cells was evaluated under fluorescence microscopy. The melanoma tumor model was established by subcutaneous implantation of B16-F10 cells into the right flank of the female C57BL6/J mice (n= 4 / group). When the tumors reached approximately 400-500 mm3, mice were randomly assigned to receive intratumoral injection of 10 μg of free EGFP-mRNA or EGFP-mRNA loaded on Ppoly (containing the same quantity of free mRNA) at N/P ratio (average number of nitrogen atoms on the carrier core/number of phosphate groups of mRNAs) 1:10 or PBS as control. Mice underwent serial imaging on the Kodak live animal imaging system and the fluorescence signal intensity of the tumors were quantified at 2, 4, 6, and 24 hours after the injection. Finally, encoding ovalbumin protein (OVA) mRNA free and loaded on Ppoly were injected into melanoma tumors in the mice every 4 days for total of 4 doses. Single-cell suspensions from tumor and spleen homogenates were prepared to evaluate the immune system response against the OVA-mRNA free and loaded on Ppoly, by flow cytometry after completion of the treatment. The therapeutic efficacy of treatment was compared among the different groups by tumor volume measurement over 17 days.
Results: The in-vitro results showed that transfection efficacy of the EGFP-mRNA loaded on Ppoly system was markedly improved compared to lipofectamine and free-EGFP-mRNA in both 2D and 3D cell cultures (Fig 1A). Quantitative fluorescence imaging of the tumor-bearing mice showed a time-dependent increase in the tumor fluorescence signal intensity in the group that received EGFP-mRNA loaded on Ppoly compared to free EGFP-mRNA at all the time points (P < 0.0001) (Fig 1B, C). Tumor follow-up over 17 days, showed a significant decrease in tumor size and weight in the group treated with OVA-mRNA in complexes with Ppoly compared to free OVA-mRNA, Ppoly or PBS control (P < 0.001) (Fig 2 C, D). The OVA-mRNA loaded on Ppoly resulted in an efficient adaptive immune response demonstrated by a marked increase in the majority of T-cell populations specifically the CD8+ T-cells in the spleen and tumor tissues as compared to the free OVA-mRNA (P < 0.01) and PBS groups (P < 0.0001) (Fig 2 E, F).
Conclusions: Our findings suggest that OVA-mRNA loaded on a new carrier could be used as a novel strategy to enhance delivery of mRNA into the melanoma cancer cells and thereby improves the antitumor cytotoxic immune-mediated response and treatment outcomes. The EGFP-mRNA fluorescence imaging was used as an effective method to guide and confirm the mRNA delivery. This new theranostic approach could be used in the future to enhance the efficacy of cancer therapeutics.