Abstract
P274
Introduction: 2-[18F]Fluoro-4-borono-phenylalanine (FBPA) is an important PET tracer for the determination of Boron Neutron Capture Therapy (BNCT) applicability. Nowadays [18F]FBPA is mostly produced from [18F]F2 gas. We previously reported a novel [18F]FBPA synthesis methods from [18F]HF which is produced by 18O(p, n)18F nuclear reaction.
While, a nuclear reactor was formerly needed for a neutron source in BNCT. A cyclotron neutron generation system was recently established. Due to this change, BNCT has possibility to expanding all over the world. As BNCT expands, we need more [18F]FBPA radioactivity in one synthesis. Since nuclear reaction yield is larger in 18O(p, n)18F nuclear reaction than 20Ne (d, α)18F. We previously reported a novel [18F]FBPA synthesis from [18F]F- which is produced by 18O(p, n)18F nuclear reaction. In this study, we tried to adopt this method for automatic synthesizer.
Methods:
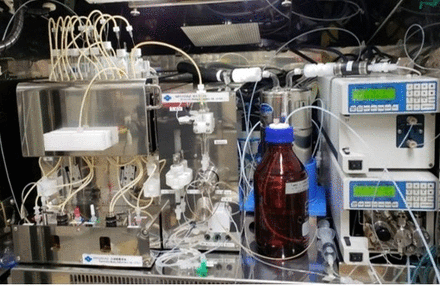
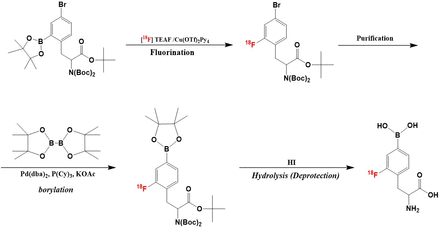
We used a modified type of MPS-200 Aβ (Sumitomo Heavy Industry, Tokyo, Figure 1) as the automatic synthesizer. The synthesis scheme is shown in Scheme 1. The first step is 18F-fluorination in the first reaction vessel. In this step, 7 mg of pinacol borane precursor and 20 mg of copper reagent were used for [18F]fluorination step in first reaction vessel. The reaction condition consists of 80-degree reaction temperature and 20 min reaction time in a mixture (1/2) of n-butanol (n-BuOH) and dimethyl acetamide (DMA) mixture (1/2). After the fluorination reaction, the reaction mixture was passed through silica gel cartridge column to remove the copper reagent and transferred to second reaction vessel. In the borylation step, we used 50 mg of bis (pinacolato)diboron, 6.0 mg of bis(dibenzylidene acetone)palladium(0), 6.0 mg of tricyclohexyl phosphine and 5.0 mg of potassium acetate in dimethy formamide (DMF). The reaction temperature was 110-degree and the reaction time was 20 min. Then, the reaction mixture was passed through a tC18 cartridge and moved to third reaction vessel. Hydroiodic acid was added to reaction mixture for deprotection. The reaction condition of this step was 100 degree in reaction temparature and reaction time was 5 min. A crude mixture was purified by HPLC. Finally, [18F]FBPA fraction was collected after HPLC purification of the crude mixture. Radiochemical purity (RCP) and molar activity (MA) were determined by HPLC. The concentrations of n-BuOH, DMA and DMF were measured with gas chromatography.
Results: The radiochemical yield of [18F]FBPA from [18F]HF was more than 10% (non-decay collected). Total synthesis time from the end of bombardment was about 110 min. RCP and MA of [18F]FBPA were more than 90% and 1,000 GBq/μmol, respectively. Each concentration of n-BuOH, DMA and DMF was less than 100 ppm. About 9 GBq of [18F]FBPA was produced by our method with 60 min and 50 microA irradiation using 12 MeV cyclotron.
Conclusions: We succeeded in synthesizing [18F]FBPA from [18F]HF using an automatic synthesizer that can be used in clinical situations.