Abstract
242298
Introduction: Cerebral blood flow (CBF) and blood-brain barrier (BBB) permeability assessment are important hemodynamic parameters in neurological conditions such as dementia. Quantification of CBF using positron emission tomography (PET) and [15O]H2O has emerged as the gold standard measure of cerebral perfusion. Aside from measuring perfusion, this technique has also been identified as a potential index of BBB permeability with an increase in [15O]H2O extraction into an altered brain. However, at higher flow rates, [15O]H2O brain extraction is non-linear, underestimating CBF measurement, and consequently, BBB permeability assessment becomes inaccurate. On the other hand, [11C]butanol extraction is almost unity and can be used to estimate the true extraction of [15O]H2O enabling a more accurate measure of CBF and BBB permeability. However, the latest method for [11C]butanol synthesis has a long production time (37 mins) and was conducted on a custom synthesizer. Furthermore, all available radiosyntheses require ethanol in the final product, which could confound [11C]butanol image interpretation. We here present an optimized [11C]butanol radiosynthesis on a commercially available synthesizer without ethanol in the final product.
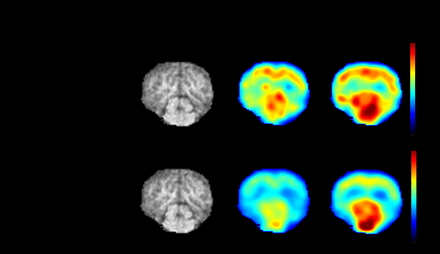
Methods: We employed a flow chemistry, captive solvent approach to reduce synthesis time and increase efficiency. This was done using a GE TRACERlab FxCPro synthesizer. [11C]CO2 generated from the 14N(p,α)11C nuclear reaction was passed through a polyethylene tube impregnated with propylmagnesium chloride. The resultant [11C]butyrate was reduced using 1 M LiAlH4 in tetrahydrofuran (THF) or 0.5 M diglyme (0.2 mL for both) while simultaneously flushing the tube's contents into a reaction vessel. After stirring for 1 min, the crude reaction was quenched with 3 M HCl (1.3 mL). [11C]butanol was isolated from the reaction mixture using high-performance liquid chromatography (HPLC) or two stacked HLB cartridges with phosphate-buffered saline or ethanol, respectively. Preliminary porcine imaging was conducted with [15O]H2O and [11C]butanol via a hybrid PET/MRI scanner, enabling a simultaneous MRI acquisition. Blood flow images were generated using a one-tissue compartment model, and brain radioactivity concentration was expressed as a standardized uptake value.
Results: With both THF and diglyme as carrier solvent for LiAlH4, activity transfer into the reactor was successful. Purification on HLB was unsuccessful for both solvents. Following seven runs with HPLC purification, [11C]butanol was produced ethanol free in a 6.0 ± 2.9 decay uncorrected yield and radiochemical purity of 97.0 ± 1.3 in 28.0 ± 1.8 minutes. The CBF measured with [11C]butanol is well within the range of value measured with [15O]water with a blood flow of 51.4 and 43.1 ml/min/100g respectively.
Conclusions: [11C]butanol has been prepared in sufficient yield for clinical imaging using a widely available commercial automated synthesizer free from ethanol and GMP-ready. This has been used with [15O]water in pigs for complementary CBF measurement. Studies repeating this BBB integrity assessment in a porcine model of altered BBB are underway to be followed by clinical translation. Also, as MRI is more widely available globally compared to PET, we will use our hybrid PET/MR system to validate an MRI technique of BBB integrity against this PET gold standard to unlock global neuroimaging utility.