Abstract
P1240
Introduction: V/Q scintigraphy is an important tool for diagnosing pulmonary embolism (PE). The characteristic finding in PE on V/Q scintigraphy is a mismatch between ventilation and perfusion, with areas of hypoperfusion on the perfusion image corresponding to normal areas on the ventilation image. This mismatch reflects obstruction of the pulmonary arteries by an embolus. V/Q scintigraphy is underutilized at present, in part due to lengthy acquisition times compared to x-ray computer tomography pulmonary angiography (CTPA). One way to reduce acquisition times is to make use of deep learning image enhancement by artificial enhancement of short acquisition/low count images, thereby increasing clinical throughout and lessening patient discomfort. The objective of this study was to apply artificial intelligence techniques through count enhancement of low-count SPECT projection data paired with their corresponding high-count planar images.
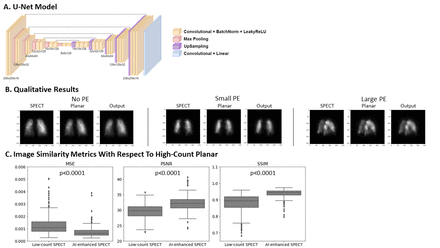
Methods: Patients. We retrospectively included 299 patients from The Ottawa Hospital who underwent V/Q scans for suspicion of PE between Sept 2019 and Mar 2022. Only perfusion images using 99m-Tc MAA were used in this study. Image Acquisition. Perfusion images of the anterior (ANT), posterior (POST), right and left posterior oblique (RPO, LPO), and right and left anterior oblique (RAO, LAO) projections were acquired with 8 machines by 2 vendors until a total of 600K counts were recorded in each projection using 256×256 matrix. Planar acquisition was on average 120.0±54.7s. SPECT acquisition followed immediately with 128 projections and 8s per stop and 128×128 matrix. Image pre-processing. Image intensity values were normalized to be between 0 and 1. SPECT-planar pairing was determined automatically by choosing the SPECT projection co-registered to a given planar projection that yielded the highest Pearson correlation coefficient. Model construction. To perform artificial count enhancement, we trained a U-Net, a variation of convolutional neural network (CNN), on paired low-count SPECT projections against high-count planar images as labels (Fig 1A). Model training. Training, validation, and test sets for model training and evaluation were constructed with a 60:20:20 split using a mean squared error (MSE) loss function. We employed early stopping and learning rate scheduling to improve convergence and reduce overfitting. Model evaluation. To assess whether AI-enhanced low-count SPECT projections were more similar to high-count planars than the original low-count SPECT projections, we calculated MSE, peak signal to noise ratio (PSNR), and structural similarity index measure (SSIM). Image similarity metrics were compared with paired t-tests.
Results: The U-Net model trained for 67 epochs and showed little overfitting, with a train, validation, and test MSE of 7.26x10-4, 7.76x10-4, and 7.21x10-4, respectively. By visual inspection, we show that subsegmental and segmental perfusion defects can still be discerned after enhancement and that no new defects are introduced, demonstrating that diagnostic information is preserved while reducing scan acquisition by a factor of approximately 15 (Fig 1B). The count-enhanced images appear smoother and with less counting noise. On average, from the low-count to AI-enhanced SPECT projections, the MSE changed by -5.39 ± 4.22 (x10-4), PSNR by 2.53 ± 1.49, and SSIM by 0.0582 ± 0.034 (all p<0.001), demonstrating that the artificially enhanced output is closer to the high-count planar (Fig 1C).
Conclusions: The proposed U-Net model appears to be promising for artificially enhancing counts lung scintigraphy to reduce scan acquisition times and to potentially generate pseudo planar images from SPECT data. Future works will incorporate perceptual and adversarial-based losses to improve image similarity with respect to high-count planar images.