Abstract
P107
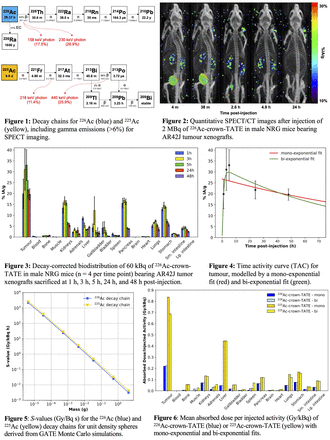
Introduction: 226Ac (t1/2 = 29 h ) has been proposed as a potential surrogate isotope for the development of 225Ac (t1/2 = 9.9 d) radiopharmaceuticals for targeted alpha therapy (TAT). 226Ac emits two gamma photons (158 keV and 230 keV) which are ideal for quantitative imaging with single photon emission computed tomography (SPECT). Beyond its imaging capabilities, 226Ac acts as an in vivo generator of 226Th (t1/2 = 30.6 m) which, through its rapid decay chain, emits four high energy alphas (6.3 – 7.7 MeV) via short-lived progeny (t1/2 = 164 μs – 38 s). The therapeutic potency of 226Ac is theoretically comparable to the four alpha emissions from the 225Ac decay chain. The primary benefits of 226Ac as a theranostic isotope is the direct imaging of 226Ac itself to assess biodistribution and its progeny’s short half-lives, minimizing the toxicities caused by progeny migration from the targeted site. In this work, we evaluate and compare the radiation dosimetry of a preclinical matched 225/226Ac-radiopharmaceutical using in vivo quantitative SPECT imaging and ex vivo biodistribution.
Methods: 226Ac activity was produced via high energy (480 MeV) proton spallation of a uranium carbide target and mass separated in the Isotope Separator and Accelerator (ISAC) facility at TRIUMF (Vancouver, Canada). Crown-TATE was labelled with 226Ac to target neuroendocrine tumours in male NRG mice bearing AR42J xenografts. Mice were injected with [226Ac]Ac-crown-TATE for SPECT imaging studies (2 MBq; n = 2) and for biodistribution studies (60 kBq; n = 20). For the imaging study, scans were acquired with the VECTor microSPECT/CT (MILabs, Netherlands) with an extra ultra high sensitivity collimator. Images were acquired dynamically at 0 – 1 h post-injection (p.i.) and statically at 2.5 h, 5 h, and 24 h p.i. SPECT images were reconstructed from the 158 keV and 230 keV photopeaks, and corrected for decay, scatter, and attenuation. Calibration factors were used to generate quantitative SPECT images. Volumes of interest were aligned with CT images and activity concentrations were measured in tumours and organs of interest. For the biodistribution study, mice were sacrificed at 1 h, 3 h, 5 h, 24 h, and 48 h p.i. (n = 4 per time point) for biodistribution measurements via gamma counter. Time activity curves (TACs) were fit with both mono-exponential and bi-exponential decay models to calculate the time integrated activity. S-values were derived from GATE-based Monte Carlo simulations of the absorbed dose in unit density spheres throughout the 226Ac and 225Ac decay chains. Assuming identical biodistribution and kinetics due to element-equivalence, radiation dosimetry was derived for both 225Ac-crown-TATE and 226Ac-crown-TATE through their respective physical half-lives and S-values.
Results: We present quantitative SPECT images of the in vivo biodistribution of 226Ac-crown-TATE, which show agreement within 8% of ex vivo biodistribution measurements in the tumours and kidneys. 226Ac-crown-TATE biodistribution studies demonstrated high uptake (>30 %IA/g at 5 h p.i.) and retention (t1/2, bio = 65 h) in tumours with low uptake and fast clearance in non-target organs. The mono-exponential decay model was appropriate for most organs. However, TAC fitting improved with the more complex bi-exponential decay model for the tumours and kidneys. The mean absorbed dose per injected activity to tumours was 0.223 Gy/kBq for 226Ac-crown-TATE and 0.687 Gy/kBq for 225Ac-crown-TATE. 226Ac-crown-TATE exhibited higher tumour-to-blood, tumour-to-kidney, and tumour-to-liver dose ratios than 225Ac-crown-TATE, suggesting lower risk of damaging clearance organs.
Conclusions: This study highlights the theranostic potential of 226Ac as a standalone therapeutic isotope in addition to its usefulness in assessing dosimetry for matched 225Ac-radiopharmaceuticals. Future work will investigate therapy and toxicity studies in mice tumour models to further explore the therapeutic potentials of 226Ac-radiopharmaceuticals.