Visual Abstract
Abstract
The purpose of this study was to determine the impact of [18F]FDG PET/CT on the initial staging, restaging, clinical management, and outcomes of patients with soft-tissue and bone sarcomas. Methods: This single-arm, prospective multicenter registry enrolled 304 patients with 320 [18F]FDG PET/CT scans (November 2018 to October 2021). Eligibility included the initial staging of a grade 2 or higher or ungradable soft-tissue or bone sarcoma, with negative or equivocal findings for nodal or distant metastases on conventional imaging before curative-intent therapy, or restaging of patients with a history of treated sarcoma with a suspicion or confirmation of local recurrence or limited metastatic disease who were being considered for curative-intent or salvage therapy. The presence of local recurrence or metastases on [18F]FDG PET/CT was recorded. Clinical management after [18F]FDG PET/CT compared with pre–[18F]FDG PET/CT planned management and quantitative metabolic tumor parameters (SUVmax, metabolic tumor volume, total lesion glycolysis) were correlated with the outcome data for 171 patients. Results: At the initial staging, [18F]FDG PET/CT detected metastases in 17 of 105 patients (16.2%) with no metastases on conventional work-up and confirmed metastases in 44 of 92 patients (47.8%) with equivocal findings for metastases. At the time of restaging, [18F]FDG PET/CT detected local recurrence in 37 of 123 patients (30.1%) and distant metastases in 71 of 123 patients (57.7%). Overall, the change in treatment intent and treatment type was recorded in 64 of 171 cases (37.4%) and 56 of 171 cases (32.8%), respectively. The presence of metastases on [18F]FDG PET/CT was associated with shorter progression-free survival at the initial staging (P = 0.04) and shorter overall survival at the time of recurrence (P = 0.002). All quantitative metabolic tumor parameters correlated with progression-free survival and overall survival. Conclusion: [18F]FDG PET/CT frequently detects additional sites of disease compared with conventional imaging in patients with sarcomas that were being considered for curative-intent or salvage therapy. This increased detection impacts the clinical management in a third of patients referred for initial staging or presumed limited recurrence after primary therapy. The presence of metastases on [18F]FDG PET/CT is associated with poorer outcomes.
Sarcomas are uncommon malignancies accounting for less than 1% of adult solid tumors. A recent comprehensive review including 78,527 patients in the United States showed that the most common subtypes are soft-tissue sarcomas (STSs), with more than 50 distinct histologic subtypes, and that most tumors are high-grade and at least 5 cm at diagnosis. These tumors are a heterogeneous group, with variable outcomes and an overall 5-y cause-specific mortality rate of 28.6% (1). The management of sarcomas is complex and requires a multidisciplinary approach. Approximately 10%–20% of patients present with metastatic disease, with metastatic patterns varying by tumor subtype. When the tumor is localized, treatment involves surgical resection with or without neoadjuvant therapy (2). However, treatment options are usually limited to systemic therapies in patients who develop metastases, except for patients with limited or isolated metastases that can be completely removed or ablated (2,3). Reported survival rates are generally better after surgical resection of metastases, with metastasectomy identified as the most important prognostic factor for survival on multivariate analysis and with long-term survival possible for patients with prolonged disease-free intervals and those in whom complete resection of pulmonary and extrapulmonary metastases is possible (3,4).
Disease extent, at baseline and at the time of recurrence after primary therapy, is vital for optimal therapy planning. Radiography, CT, and MRI are the imaging modalities of choice to delineate local tumor extent, depending on the specific primary tumor site. The initial imaging work-up and surveillance are tailored according to the subtype of the tumor and its propensity for specific metastatic sites. The National Comprehensive Cancer Network guidelines (version 2.2021) recommend CT or PET for assessment of regional lymph node metastases for angiosarcoma, clear cell sarcoma, epithelioid sarcoma, rhabdomyosarcoma, and synovial sarcoma and MRI or CT of the abdomen and pelvis for angiosarcoma, epithelioid sarcoma, and myxoid or round cell liposarcomas. Similarly, the guidelines recommend brain MRI for staging of angiosarcoma, alveolar soft-part sarcoma, and cardiac sarcoma and spine MRI for myxoid or round cell liposarcoma because of the risk of metastases to the brain and spine, respectively (5).
There are conflicting reports on the role of [18F]FDG PET/CT in the staging of sarcomas, with the European Society of Medical Oncology, National Comprehensive Cancer Network guidelines, and American College of Radiology appropriateness criteria citing PET/CT as optional for the initial staging of STS and bone sarcoma (BoS) (5–7). To obtain further real-world data on the added value of [18F]FDG PET/CT in BoS and STS, the Ontario PET registry in sarcoma was launched. Our hypothesis was that [18F]FDG PET/CT will detect additional metastases or sites of recurrence when compared with conventional imaging and that this increased detection will influence patient management. The primary aim of the current Ontario PET registry trial was to measure the impact of [18F]FDG PET/CT on the initial staging, restaging, clinical management, and outcomes of patients with BoS and STS as an adjunct to conventional imaging (CT with or without MRI). Secondary aims included determining whether an association exists among findings on [18F]FDG PET/CT, metabolic tumor parameters, and clinical outcomes.
MATERIALS AND METHODS
The Ontario PET registry for sarcomas is a multicenter prospective registry study funded by the Ontario Ministry of Health. Data were collected by Ontario Health–Cancer Care Ontario (OH-CCO) as a continuation of studies initiated by the Ontario Steering Committee for PET Evaluation. This registry was created to strengthen existing evidence for the use of PET/CT in patients with BoS and STS as an adjunct to the standard staging procedures, which include clinical and conventional radiologic investigations.
Patients meeting the inclusion criteria are referred to the PET registry by oncologists and surgeons treating sarcoma. Data were collected through OH-CCO. Analysis and presentation of the results of the current registry were conducted in accordance with OH-CCO’s designation as a prescribed entity for the purposes of section 45(1) of Ontario’s Personal Health Information Protection Act from 2004. As a prescribed entity, OH-CCO is able to collect personal health information for the purpose of analysis or compiling statistical information with respect to the management, evaluation, or monitoring of; the allocation of resources to; or planning for all or part of the health system, including the delivery of services. When planned analyses are assessed as compliant for these purposes, separate institutional research ethics board approval or patient consent is not required. OH-CCO’s information management practices are reviewed on a triennial basis by the Information and Privacy Commissioner of Ontario.
Inclusion Criteria
The inclusion criteria for this registry were as follows. The patient had to be at least 18 y old with intermediate- or high-grade (grade 2 or higher) or ungradable STS or BoS and with negative or equivocal findings for metastases on conventional imaging before curative-intent therapy, or the patient had to have a history of treated sarcoma with suspected or confirmed recurrent disease—either local tumor recurrence or limited metastatic disease—and to be under consideration for curative-intent or salvage therapy. Limited metastatic disease was defined as metastatic disease that would potentially be amenable to surgical resection or other ablative therapy. Conventional staging procedures (contrast-enhanced CT with or without MRI) were chosen by the treating oncologist depending on the specific tumor type and completed before inclusion in the registry.
Study Procedures
Patients were scanned at 1 of 9 PET/CT centers across the province of Ontario, Canada, between November 1, 2018, and October 31, 2021. Pre–[18F]FDG PET/CT patient treatment intent (curative, palliative) and a management plan if [18F]FDG PET/CT was not performed were collected on dedicated case report forms. Conventional imaging work-up before PET (CT with or without MRI) was tabulated. PET scans were obtained on integrated PET/CT scanners using standard local PET imaging protocols. These included an unenhanced low-dose CT scan preceding a PET image acquisition from the top of the skull to the upper thighs or to the feet in the case of lower-extremity tumors, with the patient in the supine position. [18F]FDG PET/CT was interpreted by a local nuclear medicine physician/radiologist who recorded the [18F]FDG avidity of the primary tumor or local recurrence (as relevant) and the sites of metastatic disease on [18F]FDG PET/CT and documented the results on dedicated data collection sheets. The presence or absence of metastases, sites of metastases, and observations equivocal for metastases on CT and [18F]FDG PET/CT, as determined by the interpreting physician, were documented and compared.
Post–[18F]FDG PET/CT Management and Outcomes
A review of the change in clinical management after [18F]FDG PET/CT and the outcome data, including progression-free survival (PFS) and overall survival (OS), were collected from a subset of patients from 2 of the referring medical centers (University Health Network and Mount Sinai Hospital). This retrospective analysis received Institutional Research Ethics Board approvals, and the requirement for informed consent was waived. For these participants, the mode and date of conventional work-up before [18F]FDG PET/CT were recorded. Treatment intent and type of treatment provided after [18F]FDG PET/CT were recorded and compared with pre–[18F]FDG PET/CT management plans. Two reviewers determined the change in treatment intent or type by consensus, with an arbitrator if consensus could not be reached.
PFS was defined as the date from biopsy to progression among the staging cohort and the date from the [18F]FDG PET/CT scan to progression among the restaging cohort, with OS defined as the date from biopsy to death from any cause.
Quantitative [18F]FDG PET/CT parameters, including SUVmax, metabolic tumor volume (MTV), and total lesion glycolysis (TLG) defined as the product of the SUVmean and MTV, were acquired on dedicated PET/CT analysis software (Mirada XD3; Mirada Medical Ltd.). Tumors (primary tumor, local recurrence, or metastases) were autosegmented using an absolute SUVmax threshold of 2.5 with manual fine tuning, when needed, as previously described (8) (Fig. 1). One reader with 5 y of experience performed this assessment, except when tumor delineation was uncertain, in which case the assessment was performed in consensus with a second reader with 20 y of experience. The correlation between the quantitative [18F]FDG PET/CT parameters and the outcome measures was determined.
Tumor segmentation of 58-y-old man with 16.7-cm left retroperitoneal dedifferentiated liposarcoma at initial staging. Segmented primary tumor in whole-body [18F]FDG PET maximum-intensity-projection image showing SUVmax of 28.2, MTV of 1,652.3 cm3, and TLG of 13,383.6 g. BW = body weight.
Statistical Analysis
Summary statistics were presented to evaluate patient demographic, clinical, imaging, and treatment characteristics for the entire and separate cohorts. A Wilcoxon rank sum test was used to compare imaging characteristics among different groups of patients. A χ2 test or Fisher exact test was used to evaluate the correlation among findings on [18F]FDG PET/CT, change in treatment intent, and change in treatment type. The Kaplan–Meier method was performed for PFS and OS analysis between groups for different cohorts. Comparisons between cohort groups were conducted using a log-rank test. Cox proportional hazard models were conducted to evaluate the effect of imaging characteristics on PFS and OS. All statistical analyses were performed using R, version 3.6.1 (R Foundation for Statistical Computing). P values of less than 0.05 were considered to indicate statistically significant differences.
RESULTS
Demographics
There were 347 [18F]FDG PET/CT scans in the sarcoma registry obtained between November 2018 and October 2021. After exclusion of duplicate examinations and incomplete datasets, there were 320 [18F]FDG PET/CT scans of 304 patients in the final study cohort (Fig. 2), including 167 men (54.9%) and 137 women (45.1%), with a median age of 57 y (range, 20–93 y). Of the 320 [18F]FDG PET/CT scans, 197 were for initial staging of sarcoma, either STS (n = 168) or BoS (n = 29), and 123 were for restaging due to local tumor recurrence (n = 44) or limited metastases (n = 79). Thirty of 123 patients (24.4%) had histologically confirmed local tumor recurrence or metastases, with the remainder having imaging suspicion of recurrent disease.
Patient flow. Duplicate scans for same indication performed within 6 mo, with only last [18F]FDG PET/CT scan for each indication included. Asterisk represents patient flow.
Data on the change in management and outcomes, including PFS and OS, from the 2 medical centers were available for 171 patients with STS (142/171) or BoS (22/171) and referred for initial staging (n = 95) or restaging (n = 76). Conventional imaging was performed at a median (±SD) of 21 ± 20.2 d from [18F]FDG PET/CT and consisted of CT of the chest and abdomen, with or without the pelvis (n = 164); a chest radiograph (n = 7); and MRI of an extremity (n = 37), the abdomen or chest (n = 11), the spine (n = 8), the pelvis (n = 5), or the head (n = 4). There were 91 men and 80 women with a mean age of 54 ± 16.5 y (range, 19–88 y). Histologic subtypes for BoS included Ewing sarcoma (n = 10), osteosarcoma (n = 9), and chondrosarcoma (n = 3), and histologic subtypes for STS included an undifferentiated or unclassified histology (n = 43), liposarcoma (n = 30), leiomyosarcoma (n = 23), fibroblastic or myofibroblastic tumors (n = 18), vascular tumors (n = 11), rhabdomyosarcoma (n = 10), or other (n = 7). Tumor grade was available for 105 patients, with most (93/105; 88.6%) having grade 2 or 3 tumors.
Initial Staging
The detection of metastases on conventional work-up (before [18F]FDG PET/CT) and after [18F]FDG PET/CT is summarized in Table 1 for the entire cohort and for BoS and STS separately. Overall, [18F]FDG PET/CT detected metastases in 17 of 105 patients (16.2%) with no known metastases, and [18F]FDG PET/CT confirmed metastases in 44 of 92 patients (47.8%) with equivocal findings for metastases on conventional work-up. Metastases were detected on [18F]FDG PET/CT in 52 of 168 patients (30.9%) with high-grade or ungradable STS and 9 of 29 patients with BoS; the sites of metastases are summarized in Table 2.
Presence of Metastases Before [18F]FDG PET/CT (Based on Conventional Work-up) and After [18F]FDG PET/CT for STS and BoS and for Entire Cohort
Distribution of Metastatic Sites on [18F]FDG PET/CT for STS and BoS
Restaging
For patients referred for restaging at the time of confirmed or suspected local recurrence (n = 44), [18F]FDG PET/CT was positive for local tumor recurrence in 21 of 44 patients (47.7%), including in all 8 patients with histologically confirmed recurrence before [18F]FDG PET/CT; equivocal in 1 of 44 patients (2.3%); and negative in 22 of 44 patients (50%). In addition, [18F]FDG PET/CT detected distant metastases in 19 of 44 patients (43.2%) referred for suspected local recurrence and was equivocal for distant metastases in an additional 2 of 44 (4.6%). For patients referred for restaging due to confirmed or suspected limited metastases, [18F]FDG PET/CT was positive in 52 of 79 patients (65.8%), including in 16 patients with histologically confirmed metastases; equivocal in 7 of 79 patients (8.9%); and negative for metastases in 20 of 79 patients (25.3%). Furthermore, 16 of 79 patients (20.3%) and 6 of 79 patients (7.6%) with suspected limited metastases were also positive or equivocal, respectively, for local tumor recurrence on [18F]FDG PET/CT.
Management Change and Outcomes
Metabolic Tumor Parameters
Metabolic tumor data, including the SUVmax of the primary tumor, local recurrence or metastasis, MTV, and TLG, are presented in Table 3. Although SUVmax was higher for BoS, and MTV and TLG were higher for STS, differences between the groups were not significant.
Metabolic Tumor Parameters at Initial Staging and Restaging for Entire Sample and for Bone and STS Separately
Change in Management
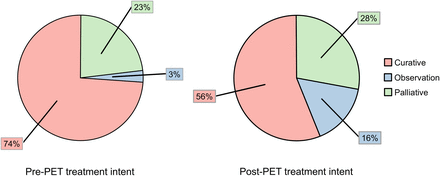
A change in treatment intent after [18F]FDG PET/CT was recorded in 64 of 171 patients (37.4%; Fig. 3), and a change in the offered treatment type was observed in 54 of 171 patients (31.2%; Fig. 4). The most common was a change mostly from planned surgery or a combined surgical plan to the initiation of systemic therapy; this change occurred in 21 of 171 patients (12.3%), including 11 of 95 patients (11.6%) in the initial staging cohort and 10 of 76 patients (13.2%) referred for restaging.
Pie charts depicting treatment intent before and after [18F]FDG PET/CT.
Pie charts depicting type of treatment before and after [18F]FDG PET/CT.
Correlation Between [18F]FDG PET/CT and Outcome Measures
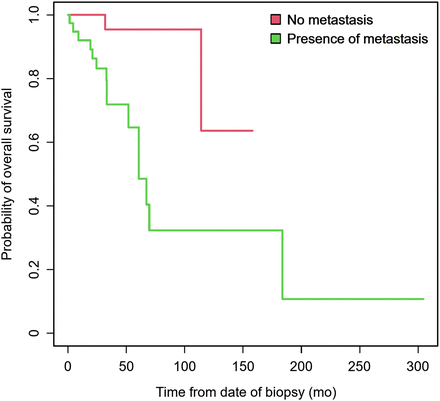
Survival data were available for 159 of 171 patients and are summarized in Table 4. At the time of the initial staging, the presence of metastases on [18F]FDG PET/CT was associated with shorter PFS (median survival, 18.3 mo [range, 7.2–54.7 mo] vs. 29.1 mo [range, 18.2 mo–not achieved] for those with and without metastases, respectively, on [18F]FDG PET/CT; P = 0.04). For patients with presumed limited disease recurrence, the presence of metastases on [18F]FDG PET/CT was associated with shorter OS (median survival, 60.7 mo [range, 51.8–183.7 mo) vs. not achieved [range, 114.1 mo–not achieved] for those with and without metastases, respectively; P = 0.002; Fig. 5).
Association Between Positive [18F]FDG PET/CT and PFS and OS
Kaplan–Meier OS curve for patients in restaging cohort, with and without metastases.
There was a significant correlation (P < 0.001) between PFS and all metabolic tumor parameters, with hazard ratios of 1.05 (95% CI, 1.03–1.07) for SUVmax, 1.0016 (95% CI, 1.0009–1.0023) for MTV, and 1.0003 (95% CI, 1.0002–1.0004) for TLG. Similarly, there was a significant correlation between OS and SUVmax, MTV, and TLG, with hazard ratios of 1.03 (95% CI, 1.01–1.06) (P = 0.016), 1.002 (95% CI, 1.001–1.003) (P < 0.001), and 1.0003 (95% CI, 1.0002–1.0005) (P < 0.001), respectively.
DISCUSSION
At the initial staging of patients with ungradable or intermediate/high-grade BoS or STS, [18F]FDG PET/CT frequently detected additional sites of disease compared with conventional imaging and confirmed metastases in nearly half of the patients with equivocal findings on conventional work-up. In the restaging setting, [18F]FDG PET/CT detected metastases in more than 40% of patients with confirmed or presumed local tumor recurrence. Tumor stage is one of the most important prognostic factors for patient outcome. The presence of metastases and the extent of metastatic disease are likely to influence the treatment approach, as was reflected in a change in treatment intent and type of treatment given after [18F]FDG PET/CT in approximately a third of the registry patients.
Lung metastases are the most common sites of distant metastatic disease, being detected in more than 20% of patients with STS, most frequently in those with a primary tumor in the extremities (9). In primary BoS, lung metastases are detected in approximately 10% to more than 40% of patients, depending on the subtype, with the highest frequency observed in those with osteosarcomas (10,11). The rate of lung metastases in our cohort is lower than the reported rates in other series (10.7% and 13.8% for STS and BoS, respectively), likely because our patient population was screened with conventional imaging before [18F]FDG PET/CT, excluding those with overt distant metastases. Similarly, we found liver metastases in nearly 9% of patients with STS on [18F]FDG PET/CT, compared with a rate of less than 7% on CT or MRI in a series of 687 patients (12). The location and frequency of metastatic disease reported in various series in the literature likely depend on the subtypes of sarcoma included and the type of imaging performed. Although [18F]FDG PET/CT is limited in the detection of lung metastases compared with CT, especially for metastases smaller than 5 mm, for which detection rates may be as low as 15% (13,14), it detects distant metastases not appreciated on conventional imaging in more than 16% of patients referred for initial staging and in more than 40% of patients referred for presumed localized tumor recurrence (Fig. 6)). [18F]FDG PET/CT provides additional important information on disease extent in these patients when used as an adjunct to CT. These findings are in line with a previous retrospective chart survey of 493 patients with high-grade BoS and STS who underwent [18F]FDG PET/CT after CT/MRI, showing an upstage rate (from M0 to M1) in 12% of cases after [18F]FDG PET/CT (15). A further study including 117 patients with BoS and STS showed higher sensitivity and overall accuracy for [18F]FDG PET/CT than for a conventional work-up for the detection of nodal and distant metastases, when compared with a reference standard of histology or imaging follow-up, with the highest accuracy being for a combination of [18F]FDG PET/CT and conventional imaging (16). From our data, we have demonstrated that the additional information provided from PET translates to a change in management in approximately a third of patients and informs prognosis. Although histologic proof of metastatic disease was not collected in our study, the presence of metastases on [18F]FDG PET/CT performed at the initial staging of patients with intermediate- or high-grade sarcoma who were being considered for curative-intent therapy was associated with shorter PFS (P = 0.04), and the presence of metastases on [18F]FDG PET/CT performed for restaging at the time of presumed limited disease recurrence was associated with shorter OS than in patients without metastases on [18F]FDG PET/CT (P = 0.002).
A 48-y-old man with shortness of breath. (A) Coronal contrast-enhanced CT of chest shows 8-cm right pulmonary mass invading right main pulmonary artery (arrow) and further filling defect in main pulmonary artery (star). (B) Corresponding [18F]FDG PET/CT coronal images show intensely metabolically active centrally necrotic mass invading right pulmonary artery (solid arrow) with separate tumor thrombus in main pulmonary trunk (dotted arrow). At surgery, 11.5-cm intimal sarcoma of pulmonary artery was resected. (C) [18F]FDG PET maximum-intensity-projection image performed 21 mo after surgery because of development of right adrenal nodule on CT (not shown) confirms right adrenal metastasis (solid arrow) and identifies metabolically active right rib 11 and right sacral lesions, consistent with bone metastases (dashed arrows). (D) Corresponding axial [18F]FDG PET/CT images (left, CT; middle, fused PET/CT; right, PET) showing right adrenal deposit (solid arrow) and metabolically active, expansile lytic lesion in right rib 11 (dashed arrow).
As reported previously in a few small-scale studies, we observed a strong correlation between SUVmax and patient outcomes, including PFS and OS (17–20). In contradiction to a previous report by Reyes Marlés et al., we showed that both MTV and TLG correlated with not only OS but also PFS. This difference from the findings of Reyes Marlés et al. may be due to differences in patient populations and methodology, as their study assessed the volumetric metabolic parameters of the primary tumor only at the time of the initial staging whereas our segmented tumor volumes included all sites of metabolically active disease, perhaps better reflecting disease extent (19). All of these metabolic tumor parameters were predictive of patient outcomes.
There are limitations to the current study. First, the type of conventional work-up before [18F]FDG PET/CT was left to the discretion of the treating oncologist or surgeon. Although this may result in a nonuniform work-up before [18F]FDG PET/CT, it is likely a reflection of the heterogeneity of sarcomas, with differences in patterns of metastatic spread or differences in the propensity of the different subtypes for local recurrence. Second, we did not collect histologic proof for the findings on [18F]FDG PET/CT or collect imaging follow-up for equivocal lesions on conventional work-up that were negative on [18F]FDG PET/CT, and it is possible that some of the lesions that were considered metastases on [18F]FDG PET/CT were false positives; however, we used actual clinical outcomes as a surrogate to validate the imaging findings. The presence of metastases on [18F]FDG PET/CT correlated with OS in this preselected population of patients who had no metastases or had equivocal findings for metastases on conventional work-up. Third, [18F]FDG PET/CT scans were interpreted locally by readers with variable experience, rather than centrally, as reflects the design of this registry study aimed to collect real-world data on the added value of [18F]FDG PET/CT in this setting in clinical practice. Finally, we do not have direct data on the impact of a change in the type of treatment given after [18F]FDG PET/CT. However, better delineation of tumor extent likely results in more appropriate patient management. Approximately 12% of patients referred for either initial staging or restaging had their treatment changed from surgery to systemic therapy. This change is most likely explained by the detection of distant metastases on [18F]FDG PET/CT, making systemic therapy the more suitable approach.
CONCLUSION
[18F]FDG PET/CT frequently detects additional sites of disease compared with conventional imaging in patients with STS or BoS who are being considered for curative-intent or salvage therapy. Increased detection impacts clinical management in a third of patients referred for initial staging or presumed limited recurrence after primary therapy. The presence of metastases on [18F]FDG PET/CT is associated with poorer outcomes.
DISCLOSURE
No potential conflict of interest relevant to this article was reported.
KEY POINTS
QUESTION: Does [18F]FDG PET/CT contribute—beyond conventional imaging procedures—to the staging and management of patients with grade 2 or higher BoS or STS at initial presentation or at the time of presumed limited recurrence?
PERTINENT FINDINGS: [18F]FDG PET/CT detected metastases in 17 of 105 patients (16.2%) with no metastases on conventional work-up at initial staging, local recurrence in 37 of 123 patients (30.1%), and distant metastases in 71 of 123 patients (57.7%) at restaging of presumed limited recurrent disease, resulting in a change in treatment intent and treatment type in approximately 1 in 3 patients.
IMPLICATIONS FOR PATIENT CARE: [18F]FDG PET/CT frequently detects disease sites beyond those seen on conventional staging procedures, frequently impacting patient management.
ACKNOWLEDGMENTS
Support for this work was provided by OH-CCO and the Ontario Ministry of Health.
Footnotes
Published online Jul. 6, 2023.
- © 2023 by the Society of Nuclear Medicine and Molecular Imaging.
REFERENCES
- Received for publication December 1, 2022.
- Revision received April 25, 2023.