Visual Abstract
Abstract
Salvage elective nodal radiotherapy (ENRT) is a treatment option for patients with biochemically persistent or recurrent prostate cancer who have lymph node metastases (LNs) after prostatectomy. Possible ENRT templates were proposed by the Radiation Therapy Oncology Group (RTOG; 2009), the PIVOTAL trialists (2015), and the NRG Oncology Group (2021). The goal of this study was to analyze the distribution of prostate-specific membrane antigen (PSMA) PET/CT–positive LNs and to compare the templates regarding their LN coverage. Methods: We analyzed the PSMA PET/CT scans of 105 patients with PET-positive LNs treated with salvage ENRT from 2014 to 2019. All LNs were mapped in an exemplary dataset, classified by region, and assessed with regard to their potential coverage by the 3 ENRT templates. The primary endpoint was the number of missed LNs. The secondary endpoint was the number of patients with full coverage. To compare the templates, a t test and McNemar test were used. Results: Three hundred thirty-five LNs were contoured (3.19 per patient; 95% CI, 2.43–3.95). Most frequently, LNs were seen in the internal iliac (n = 94, 28.1%), external iliac (n = 60, 17.9%), periaortic (n = 58, 17.3%), common iliac (n = 55, 16.4%), perirectal (n = 26, 7.8%), and presacral (n = 19, 5.7%) regions. The NRG template missed fewer LNs per patient (1.01, 31.7%) than the RTOG (1.28, 40.1%, P < 0.001) and PIVOTAL templates (1.19, 37.3%, P = 0.003). No difference was observed in the number of patients with full coverage of all LNs: 52 (49.5%) with the NRG template versus 50 (47.6%) with the RTOG (P = 0.625) and 49 (46.7%) with the PIVOTAL template (P = 0.250). Conclusion: The NRG template showed better coverage than the RTOG and PIVOTAL templates. Nevertheless, in this cohort, it would have missed almost one third of all contoured LNs and would have resulted in incomplete coverage in half the patients. This result underlines the importance of advanced imaging, such as PSMA PET/CT scans, before salvage ENRT and shows the need for further individualization of ENRT fields.
In several clinical situations, elective nodal radiotherapy (ENRT) is part of the treatment of prostate cancer (PC) patients. Possible indications for ENRT can be a high risk for lymph node metastases (LNs) in primary patients or PET-positive or pathologically confirmed LNs in postoperative patients undergoing either adjuvant or salvage radiotherapy. Regarding the benefit of adding ENRT to androgen deprivation therapy in postoperative patients with pathologically confirmed LNs, there is ample retrospective evidence for better PC-specific survival (1–3). Likewise, in patients with biochemical recurrence, the addition of ENRT leads to a better outcome than prostate bed radiotherapy with or without short-term androgen deprivation therapy (4).
In 2009, the Radiation Therapy Oncology Group (RTOG) reached a consensus for contouring the ENRT volume and published their recommendations (5). Another template was suggested by the PIVOTAL trialists in 2015 (6). Their intention was to find a compromise between the detailed RTOG recommendations, which are reproducible and easy to delineate, and the complex recommendations for free hand contouring of the Royal Marsden Hospital in London, which were known to be safe at that time. However, some publications raised concerns about incomplete coverage of LNs using the RTOG template (7–11). Likewise, an adapted version of the PIVOTAL template demonstrated seemingly insufficient coverage (12). In 2021, the NRG oncology group published another contouring guideline (13) with the intention to update the RTOG recommendations based on new LN distribution data. To our knowledge, the NRG template has thus far been evaluated only by Filimonova et al. (14). Although some of the mentioned studies were performed with conventional imaging (MRI and CT) or with different and nowadays outdated 18F-choline, 11C-acetate, and FDG PET/CT scans (7,8,15), other studies already incorporated prostate-specific membrane antigen (PSMA) PET/CT scans (9–11,14). Overall, PSMA PET/CT is currently the most sensitive imaging modality for LN detection (16,17) and outperforms standard imaging such as bone scanning, CT, and MRI, particularly at low prostate-specific antigen (PSA) levels (18–20). Until now, nodal coverage has not been directly compared among the 3 ENRT templates in the postoperative setting. Here, we analyze LN coverage applying the various templates in a postoperative PC patient cohort all staged with PSMA PET/CT scans.
MATERIALS AND METHODS
Patient Population
In 2014, PSMA PET/CT scans before radiotherapy were introduced at our institution as the standard diagnostic staging tool routinely used in PC patients. For this analysis, we included a total of 105 consecutive postoperative patients referred for salvage ENRT who had undergone PSMA PET/CT because of PSA persistence or recurrence and who had evidence of PSMA-positive regional and paraaortic LNs. Patients with additional bone metastases were included, whereas patients with additional visceral metastases or supradiaphragmatic LNs were excluded.
This retrospective analysis was performed in compliance with the principles of the Declaration of Helsinki and its subsequent amendments (21) and was approved by the local Ethics Committee of the University of Munich (approval 19-361). The requirement to obtain informed consent was waived.
PSMA Ligand and PET/CT Imaging Protocol
Pretreatment imaging was performed with 68Ga- or 18F-labeled PSMA ligand PET/CT scans in 65% (68Ga-PSMA-11) and 35% (18F-PSMA-1007, after 2018) of patients, respectively. Radiolabeling was performed according to good clinical practice as described elsewhere (22,23). A Siemens Biograph 64, a Siemens Biograph mCT, or a GE Healthcare Discovery 690 PET/CT scanner was used for PSMA PET/CT imaging. Phantom studies based on the National Electrical Manufacturers Association NU2-2001 standard were conducted to allow for pooling of scanner results. At the time of the PET scan, a contrast-enhanced diagnostic CT scan (120 kV, 100–400 mAs, dose modulation) or a low-dose CT scan (120 kV, 25 mAs) for attenuation correction was performed depending on previous CT scans and contraindications. PSMA PET/CT scans were acquired approximately 60 min after injection of the PSMA ligand. Barring any contraindications, patients were administered 20 mg of furosemide at the time of tracer injection to avoid bladder activity and to reduce radiation exposure.
Image Interpretation
PET/CT scans were interpreted by 1 nuclear medicine physician and 1 radiologist in the sense of a clinical report–based analysis. Both readers had more than 5 y of PET/CT experience. The location of each lesions was determined by a CT scan. PET-positive lesions were visually identified by 68Ga-/18F-PSMA uptake above the background level and not associated with the physiologic uptake (24).
Anatomic Mapping
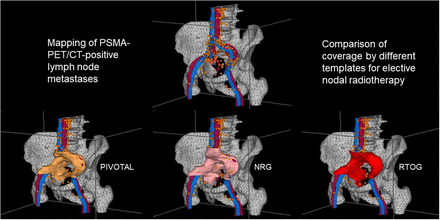
To allow systematic topographic mapping, the cross-sectional nodal atlas published by Martinez-Monge et al. (25) with small modifications (Table 1) was used. For each patient, the number and location of the PET-positive LNs were documented. Beyond summarizing these data in a table, we manually contoured each LN in a virtual patient dataset to achieve a 3-dimensional visualization of the cumulative LN distribution (Fig. 1). The Oncentra MasterPlan (version 4.5.2; Elekta) planning system was used for contouring and generating 3-dimensional images for the atlas. Moreover, every lymphatic drainage region in each patient was assessed regarding a potential geographic miss by counting LNs that would not have been treated adequately by the 3 templates (Fig. 2). A LN was considered covered if more than the half its volume was covered by the respective clinical target volume (RTOG, PIVOTAL, or NRG). Contouring was performed in accordance with the original publications (5,6,13). Main differences between the templates are pointed out in Table 2.
Distribution of LNs
CT scan dataset showing veins (blue), arteries (red), bones (gray) and all PSMA-positive LNs of patients with PSA persistence (orange).
CT scan dataset showing veins (blue), arteries (red), bones (gray), NRG ENRT volume (pink) and all PSMA-positive LNs of patients with PSA persistence not covered by NRG volume (orange).
Characteristics of and Differences in ENRT Templates
For the assessment of geographic miss, distances to relevant anatomic structures (vessels, bone, muscle, bladder, bowel) were considered, as well as the craniocaudal position in relation to vessel bifurcations or bony landmarks.
Endpoints and Statistical Analysis
The primary endpoint of this study was the potential coverage of LNs by the 3 templates. Because information about coverage was collected for every patient for every lymphatic drainage region, statistical analyses were performed on a per-patient basis. The secondary endpoint was the number of patients with full coverage of all LNs by the 3 templates. Statistical analyses were conducted using IBM-SPSS, version 26.0. To compare the templates regarding the primary endpoint, a paired-samples t test was used. Regarding the secondary endpoint, a McNemar test was used. A P value of less than 0.05 was considered significant.
RESULTS
Overall, we analyzed the PSMA PET/CT scans of 105 patients who underwent PSMA PET/CT–guided radiotherapy at our institution between 2014 and 2019 due to LN recurrence after radical prostatectomy. Patient characteristics are shown in Table 3. Indications for PSMA PET/CT scans were either biochemical recurrence (29%) or PSA persistence after surgery (71%). Median PSA at time of the PSMA PET/CT scan was 1.65 ng/mL in patients with PSA persistence and 0.65 ng/mL in patients with biochemical recurrence. Additionally, PSMA PET/CT scans revealed bone metastases and local recurrences in the prostate bed in 12% and 31% of cases, respectively.
Patient Characteristics (n = 105)
In total, 335 PSMA PET/CT–positive LNs were detected, which corresponds to 3.19 LNs per patient. The detailed distribution across the lymphatic drainage regions is shown in Table 3. Most frequently, LNs developed in the internal iliac (28%), external iliac (18%), periaortic (17%), and common iliac (16%) regions. In our exemplary CT dataset, the ENRT templates had a volume of 369 cm2 (RTOG), 375 cm2 (PIVOTAL), and 432 cm2 (NRG). In Table 1, the proportion of LNs that would not have been covered by the templates is shown for every lymphatic drainage region. The regions with the greatest differences among the 3 templates were the periaortic, common iliac, perirectal, and presacral.
The RTOG template would have missed 1.28 LNs (mean) per patient (40.1%). Use of the PIVOTAL and NRG templates would have reduced the number of missed LNs per patient to 1.19 (37.3%) and 1.01 (31.7%), respectively (Table 4). This resulted in significantly improved coverage using the NRG template compared with the RTOG (P < 0.001) and PIVOTAL templates (P = 0.003). However, the PIVOTAL template covered significantly more LNs than the RTOG volume (P = 0.028). Regarding the secondary endpoint, the NRG template would not have resulted in a significantly higher proportion of patients with complete coverage of all LNs (52 patients, 49.5%) than would the RTOG (50 patients, 47.6%; P = 0.625) or PIVOTAL templates (49 patients, 46.7%; P = 0.250 for comparison with NRG, P = 1.00 for comparison with RTOG).
Coverage of LNs
DISCUSSION
The present study shows the distribution of PSMA PET/CT–positive LNs in postoperative PC patients. There are several other choline PET/CT– or PSMA PET/CT–based analyses on the location and ENRT coverage of LNs in postoperative PC patients (9–11,15,26). The results are mostly similar. Interestingly, the choline PET/CT–derived analyses found comparably fewer internal iliac LNs while describing comparably more common iliac LNs (15,26). Moreover, 2 studies with comparably low PSA levels at the time of the PET/CT scans found fewer periaortic and more perirectal LNs (9,10).
Furthermore, the present study evaluated how many LNs would have been covered by the respective ENRT templates. Most of the mentioned studies used the RTOG template in this regard (9–11,26) and described a slightly better coverage (63%–66%) than our study (60%). First, this better coverage may be associated with lower PSA levels at the time of PET/CT scans (9,10), as lower levels are known to correlate with higher coverage because of overall lower LN numbers (9,11). Second, the better coverage is most likely because of the staging by the choline PET/CT scan (26), which is known to have a lower detection rate than PSMA PET/CT scans (27). The study of Schiller et al., which had PSA levels similar to those in the present analysis and used PSMA PET/CT scans, described an even worse coverage (complete and partial) of 48% (11). The study of De Bruycker et al. (15) using the PIVOTAL template reported a lower miss rate of 27%, which is less than in our study (37%). Admittedly, they modified the template and used a more cranial border (top of L4 instead of bottom of L5), which explains the higher coverage. Regarding the newer NRG template, Filimonova et al. (14) found that 35% of patients had LNs outside the template, whereas in our study, 51% had incomplete coverage. This difference is because of the exclusion of paraaortic LNs by Filimonova et al. When excluding patients with paraaortic LNs in the present study, we found that 39% had LNs outside the NRG template (RTOG, 41%; PIVOTAL, 42%), which is similar to Filimonova et al.
To the best of our knowledge, the present study is the first analysis with a direct comparison among the 3 templates. It shows that the PIVOTAL volume, which combines characteristics of the RTOG guideline with the experience of the Royal Marsden Hospital, covers significantly more LNs than the RTOG template and that the NRG volume, which was intended to be an updated and improved version of the RTOG volume, covers significantly more LNs than both other templates. Thus, the changes in the NRG volume improved the former RTOG volume, resulting in an overall better coverage in the present analysis. One of the key differences concerns the superior border, which extends to more cranial regions in the NRG template (aortic or caval bifurcation instead of L5/S1 interspace). Spratt et al. (7), who developed another MRI-, CT- and FDG-PET/CT based atlas in PC patients with recurrences after primary radiotherapy, found a coverage of 42% with the RTOG template, which would improve to 93% when extending the volume cranially to the L4/L5 interspace. Nevertheless, there are still lymphatic drainage regions not adequately covered by the NRG template. For example, of 335 LNs in the present analysis, there were 53 periaortic, 22 perirectal, 9 perivesical, and 9 inguinal metastases missed by the NRG template. This finding leads to the question of whether the obvious solution would be to further enlarge the ENRT volumes toward these areas. However, most likely, this solution would be at the expense of increased toxicity. Although some studies have thus far used the RTOG recommendations with a more cranial border (e.g., OLIGOPELVIS (28) or the RTOG 0924 trial [results outstanding]), there is no direct comparison evaluating a possible increase in toxicity by extending the template. Concerns about toxicity have also been the reason to exclude perirectal nodes from the NRG template against better knowledge of a potential geographic miss.
Interestingly, despite the better coverage of the NRG template, there was no significant difference among the numbers of patients with full coverage in this study. The reason may be that some patients had several LNs in areas not covered by the templates, which overestimates their statistical impact in the LN-based approach. Thus, the question remains of whether the better coverage of the NRG template really leads to more patients who are adequately treated.
Nevertheless, despite the improvement of the ENRT template by the NRG recommendations, there are still some pending questions. The most important question deals with the indication for ENRT, which is still a subject of intense discussions. Thus far, the results of recent phase 3 studies, for example, the POP-RT trial in the definitive setting (29) and—albeit before the PSMA PET/CT scan era—the SPPORT trial in the salvage setting (4), have strengthened the role of ENRT. Furthermore, in the clinical setting of PET-positive LNs, there is an ongoing discussion about the best treatment strategy. ENRT with or without a boost to the LNs seems to be associated with fewer recurrences in the adjacent LN regions at the cost of increased toxicity compared with stereotactic body radiotherapy (30). In this regard, prospective toxicity data on patients receiving ENRT with the NRG template are pertinent. In our opinion, possible further changes in ENRT fields should always be closely evaluated regarding efficacy and toxicity. Furthermore, clinical and imaging parameters should ideally be defined to allow for further individual adaptation of radiation fields. Until now, the PSA value before PET/CT was the only known parameter associated with a higher risk for LNs outside the template (9,11).
Admittedly, there are some limitations of the present analysis. First, it is based on a retrospective cohort incorporating postoperative patients with either biochemical persistence or recurrence and a relatively wide PSA range before PSMA PET/CT scans. Also including patients with high PSA values and bone metastases led to a high number of evaluated LNs but complicates the interpretation of the absolute numbers of the endpoints. In patients with lower-risk profiles, the templates would probably cover considerably more LNs. For example, when restricted to only the subgroup without bone metastases and with a PSA of less than 2.0 ng/mL (n = 57 with a mean of 2.68 LNs per patient)—similar to the cohort of the SPPORT trial (4)—the templates would have missed 39% (RTOG), 37% (PIVOTAL), and 33% (NRG) of LNs, and 56% (NRG and PIVOTAL) and 58% (RTOG) of the patients would have had full coverage. Second, the sensitivity of 18F-PSMA-1007 for LNs near the urinary bladder might be higher than that of 68Ga-PSMA-11 because of the lower urinary excretion (31). Third, the RTOG and PIVOTAL templates were designed primarily for patients in the definitive and not in the postoperative setting, whereas the present and most of the aforementioned analyses evaluated the 3 templates in a postoperative cohort in which the lymphatic drainage might have been altered because of lymphadenectomy. Fourth, manual contouring of LNs is not completely reproducible. However, using this method, we separately assessed every LN in consideration of the respective original clinical target volume recommendation.
Despite being the gold standard in the postoperative setting of PSA persistence or recurrence and showing a good specificity of 95%, PSMA PET/CT scans have a sensitivity of only 40% in detecting LNs compared with histopathologic reports (32). Thus, when considering smaller radiation fields or even stereotactic body radiotherapy, one must keep in mind that the real extent of LNs might be greater than visualized on PSMA PET/CT scans. Nevertheless, the present analysis shows that PSMA PET/CT scanning before radiotherapy is a vital component for individualizing ENRT volumes for the respective patient, with a possibly higher chance for cure.
CONCLUSION
The present study analyzed the distribution of PSMA PET/CT–positive LNs in postoperative PC patients and found a distribution similar to that of other studies. Moreover, to our knowledge, this study was the first to compare the coverage of PSMA PET–positive LNs by the RTOG, PIVOTAL, and NRG ENRT recommendations and found a significantly improved coverage by the more recent NRG template. Nevertheless, in the study population, the NRG template would have missed almost one third of all contoured LNs and would have resulted in incomplete coverage in half the patients. This result underlines the importance of advanced imaging, such as PSMA PET/CT scans, before salvage ENRT and shows the need for further individualization of ENRT fields. When ENRT volumes are enlarged, data on a possible increase in toxicity are lacking and should be prospectively collected.
DISCLOSURE
Leonie Beyer is an employee of Novartis Radiopharmaceuticals GmbH. Wolfgang Kunz has served on advisory boards for Bristol-Myers-Squibb (unrelated to the current study). No other potential conflict of interest relevant to this article was reported.
KEY POINTS
QUESTION: Which common ENRT template has the best coverage of PSMA-positive LNs in postoperative PC patients?
PERTINENT FINDINGS: LN distribution was similar to other studies. The new NRG template showed a significantly better coverage than the older RTOG and PIVOTAL templates but still resulted in incomplete coverage in half the patients in this cohort.
IMPLICATIONS FOR PATIENT CARE: The results suggest that use of the new NRG template should be recommended. The results show the importance of pretherapeutic advanced imaging, such as PSMA PET/CT scans.
Footnotes
Published online Feb. 2, 2023.
- © 2023 by the Society of Nuclear Medicine and Molecular Imaging.
REFERENCES
- Received for publication November 20, 2022.
- Revision received January 26, 2023.