Visual Abstract
Abstract
Early use of targeted radionuclide therapy to eradicate tumor cell clusters and micrometastases might offer cure. However, there is a need to select appropriate radionuclides and assess the potential impact of heterogeneous targeting. Methods: The Monte Carlo code CELLDOSE was used to assess membrane and nuclear absorbed doses from 177Lu and 161Tb (β−-emitter with additional conversion and Auger electrons) in a cluster of 19 cells (14-μm diameter, 10-μm nucleus). The radionuclide distributions considered were cell surface, intracytoplasmic, or intranuclear, with 1,436 MeV released per labeled cell. To model heterogeneous targeting, 4 of the 19 cells were unlabeled, their position being stochastically determined. We simulated situations of single targeting, as well as dual targeting, with the 2 radiopharmaceuticals aiming at different targets. Results: 161Tb delivered 2- to 6-fold higher absorbed doses to cell membranes and 2- to 3-fold higher nuclear doses than 177Lu. When all 19 cells were targeted, membrane and nuclear absorbed doses were dependent mainly on radionuclide location. With cell surface location, membrane absorbed doses were substantially higher than nuclear absorbed doses, both with 177Lu (38–41 vs. 4.7–7.2 Gy) and with 161Tb (237–244 vs. 9.8–15.1 Gy). However, when 4 cells were not targeted by the cell surface radiopharmaceutical, the membranes of these cells received on average only 9.6% of the 177Lu absorbed dose and 2.9% of the 161Tb dose, compared with a cluster with uniform cell targeting, whereas the impact on nuclear absorbed doses was moderate. With an intranuclear radionuclide location, the nuclei of unlabeled cells received only 17% of the 177Lu absorbed dose and 10.8% of the 161Tb dose, compared with situations with uniform targeting. With an intracytoplasmic location, nuclear and membrane absorbed doses to unlabeled cells were one half to one quarter those obtained with uniform targeting, both for 177Lu and for 161Tb. Dual targeting was beneficial in minimizing absorbed dose heterogeneities. Conclusion: To eradicate tumor cell clusters, 161Tb may be a better candidate than 177Lu. Heterogeneous cell targeting can lead to substantial heterogeneities in absorbed doses. Dual targeting was helpful in reducing dose heterogeneity and should be explored in preclinical and clinical studies.
Targeted radionuclide therapy (TRT) uses radiopharmaceuticals that bind to tumors to deliver targeted radiation. Significant successes have been recorded in recent years (1), notably in metastatic neuroendocrine tumors with the radiolabeled somatostatin analog 177Lu-DOTATATE (2) and in castration-resistant metastatic prostate cancer with 177Lu-PSMA (3,4). Currently, TRT is used mainly in advanced metastatic disease. There is, however, significant interest in moving TRT earlier with the hope of achieving cure, following the example of 131I therapy in thyroid cancer (5,6).
Distant metastases start with the shedding of circulating tumor cells (CTCs) from the primary site into the blood, where CTCs are found as single cells or as clusters (2 to >50 cells) (7,8). CTC clusters can be homotypic (made of cancer cells only) or heterotypic (associating with other cells, such as macrophages, neutrophils, platelets, and fibroblasts) (7,8). CTC clusters have 20- to 100-fold greater metastatic potential than single CTCs because of an increased ability to survive within the bloodstream, evade the immune system, and initiate metastatic lesions at distant sites (8). Their presence in blood is generally associated with unfavorable clinical outcomes (7,8).
The use of TRT to eradicate CTC clusters, micrometastases, or minimal residual disease (7–9) is highly relevant. However, currently used radionuclides, emitting medium-energy (177Lu, 131I) or high-energy (90Y) β− particles, are suboptimal for TRT of tiny tumor lesions, as most of the energy will be deposited outside the lesions (10–12). Other radionuclides are now being explored, including more suitable β− emitters, α-emitters, and Auger electron emitters.
161Tb has relevant properties for TRT, including for small lesions (12–17). Indeed, in addition to a β− spectrum (mean energy, 154 keV, comparable to 177Lu 133 keV), 161Tb emits multiple low-energy conversion electrons and very low-energy Auger electrons that confer an advantage to 161Tb over 177Lu at up to about 30 μm from the decay site (14). As radiolanthanides, 161Tb and 177Lu share similar chemistry (13,17). The 161Tb half-life (6.96 d) is close to that of 177Lu (6.65 d). Like 177Lu, 161Tb emits photons useful for imaging. Moreover, 2 isotopes (155Tb, 152Tb) offer the possibility for SPECT or PET imaging before therapy (13,14). The superiority of 161Tb over 177Lu has been documented in preclinical studies (17). Also, a recently published case report provided proof-of-concept clinical evidence of the therapeutic potential of 161Tb-PSMA-617 in prostate cancer (18).
We previously showed that, when all cells in a tumor cluster are targeted, 161Tb delivered 2- to 3-fold higher nuclear absorbed doses than 177Lu (15). However, cell targeting can be nonuniform, such as when some cells lose the target that allows the radiopharmaceutical (radioligand) to be recognized or attached. This nonuniformity should lead to heterogeneity in absorbed dose (19–21). Thus, we here modeled situations of uniform and nonuniform targeting with 177Lu and 161Tb within tumor clusters. We assessed nuclear absorbed doses in labeled and unlabeled cells (19,20,22). We also assessed absorbed doses to the cell membrane, another important target for TRT (23,24). Finally, as multitargeting is now widely used in oncology to counter tumor heterogeneity (25) and has been suggested in TRT (1,26,27), we assessed through Monte Carlo modeling whether a second targeting radiopharmaceutical, the distribution of which is independent of the first, may reduce absorbed dose heterogeneities.
MATERIALS AND METHODS
We assessed absorbed doses from simulations performed with CELLDOSE (11,28). 177Lu and 161Tb electron emissions were taken from the International Commission on Radiological Protection publication 107 (29). The whole β− spectrum was considered, as well as all conversion and Auger electrons with a probability greater than 0.01%. The tumor cluster consisted of 19 cells with a central cell surrounded by 6 immediate neighbors and a second layer of 12 neighbors (Fig. 1A). Each cell had a 14-μm diameter, a 10-nm-thick membrane, and a centered nucleus of 10 μm (Fig. 1B). Three distributions of the radionuclide were investigated: cell surface, intracytoplasmic, and intranuclear. We assessed absorbed doses to cell nuclei and cell membranes (with an intranuclear radionuclide location, only nuclear absorbed doses were assessed). For each cell, we individualized the self-dose and the cross-dose from surrounding cells (22).
Tumor cluster model. In present study, hatched cells (4/19) contained no activity. (Adapted from (15).)
CELLDOSE is a homemade Monte Carlo track-structure code for simulating the transport of electrons in water, based on differential and total interaction cross sections describing the elastic scattering, electronic excitation, and ionization (11,12,14). This code has been validated against experimental data and benchmarked against various codes. Photons are neglected. This is also the case in other studies on cell clusters, given the negligible energy deposited by x and γ photons (20,22). The energy transferred from primary and secondary electrons to the medium is scored event by event until their kinetic energy falls below 7.4 eV (i.e., the excitation threshold of the water molecule in liquid phase), and residual energy is assumed to be deposited locally (11). This ability of CELLDOSE to follow electrons until a low-energy level allows assessing absorbed dose in the 10-nm-thick cell membrane. The uncertainty associated with the energy deposits of subcutoff electrons (<7.4 eV) becomes relevant only when considering subnanometer structures (30).
Because electron energy per decay differs between 177Lu (147.9 keV) and 161Tb (202.5 keV), simulations were normalized considering that 1,436 MeV were released per labeled cell from either cell surface, cytoplasm, or nucleus (9,709 decays of 177Lu or 7,091 decays of 161Tb). The figure of 1,436 MeV was selected considering cell volume (1,436 μm3) and 1 MeV released per cubic micrometer (12,14,15).
We considered situations of uniform cell targeting, as well as situations of nonuniform targeting in which 4 of 19 cells in the clusters were unlabeled (hatched cells in Fig. 1A).
Finally, to assess the usefulness of dual targeting in counteracting dose heterogeneity from nonuniform targeting, we performed for each situation 2 simulations, one mimicking the first radiopharmaceutical and the other mimicking a second radiopharmaceutical. Both radiopharmaceuticals are labeled with the same radionuclide, either 177Lu or 161Tb, and distribute to similar compartments (cell surface, intracytoplasmic, or intranuclear compartment). However, they aim at 2 different targets. The expression of these targets on tumor cells are independent of one another. With each radiopharmaceutical, 4 cells are unlabeled, their position in the cluster being randomly selected. Thus, after successive simulations with the 2 radiopharmaceuticals, a cell can be double-labeled, single-labeled, or unlabeled. We took the mean absorbed dose from the 2 simulations.
RESULTS
Absorbed Doses Delivered by 177Lu and 161Tb When All Cells in the Cluster Are Labeled
When the radionuclide is at the cell surface, the absorbed doses to the cell membranes are high (177Lu, 38–41 Gy; 161Tb, 237–244 Gy), with a large contribution from self-dose (Table 1), whereas nuclear absorbed doses are comparatively low (177Lu, 4.7–7.2 Gy; 161Tb, 9.8–15.1 Gy) (Table 2). The dose to the membrane is heterogeneous, consisting of multiple impact points. Indeed, if we consider that local interactions around a decay point would occur mostly in a cylinder of 10-nm height (membrane thickness) and 10-nm radius, the ratio between the volume of this cylinder and that of the whole membrane is 5.1 × 10−7. So, even after considering all decays (177Lu, 9,709; 161Tb, 7,091), local interactions involve 0.5% or less of the cell membrane. Also, as measured with CELLDOSE, the absorbed dose to a cylinder (10-nm height, 10-nm radius) from a decay occurring at its surface is extremely high (177Lu, 3,585 Gy; 161Tb, 37,555 Gy).
Absorbed Doses from 177Lu and 161Tb to Membrane of Cells Within Tumor Cluster,* Considering Various Distributions of Radionuclide
Absorbed Doses from 177Lu and 161Tb to Nucleus of Cells Within Tumor Cluster,* Considering Various Distributions of Radionuclide
With the radionuclide in a intracytoplasmic location, absorbed doses to the cell membranes (177Lu, 6.7–9.4 Gy; 161Tb, 16.9–20.1 Gy) are comparable to nuclear absorbed doses (177Lu, 5.8–8.3 Gy; 161Tb, 12.9–17.9 Gy) (Tables 1 and 2). Finally, when the radionuclide is in an intranuclear location, nuclear absorbed doses are high (177Lu, 13.5–15.7 Gy; 161Tb, 43.1–47.8 Gy), with a large contribution from self-dose (Table 2).
In Figure 2, we plot membrane and nuclear absorbed doses to the central cell of the cluster for the different configurations. Absorbed doses delivered by 161Tb are consistently higher than those delivered by 177Lu. The highest 161Tb/177Lu absorbed dose ratio (∼6.1) is for cell membranes when the radionuclide is on the cell surface (Table 1).
Absorbed doses to central cell of cluster from 177Lu (blue) and 161Tb (red).
Effect of Heterogeneous Cell Targeting on 177Lu and 161Tb Absorbed Doses
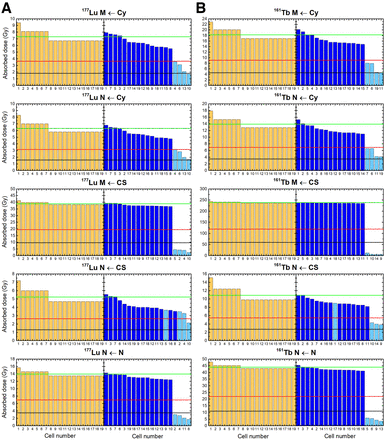
Figure 3 shows absorbed doses delivered by 177Lu (Fig. 3A) and 161Tb (Fig. 3B) in situations of uniform targeting and heterogeneous targeting. The mean absorbed dose is when all 19 cells are targeted, with doses to individual cells depending on their position within the cluster. The figure also indicates 50% of this mean dose (0.5D) and 25% (0.25D). When 4 cells are unlabeled, the cluster contains only 79% of the total activity. Absorbed doses to labeled cells are lower than with uniform targeting because of a reduced cross-dose. The impact on unlabeled tumor cells is more pronounced and is dependent mainly on the specific configuration of radionuclide location or target.
Absorbed doses from 177Lu and 161Tb to cell membranes and nuclei for situations of uniform cell targeting (amber) and nonuniform targeting (blue, with dark blue corresponding to labeled cells and light blue to 4 unlabeled cells) and for various distributions of radionuclide. Green line represents mean absorbed dose for uniform targeting; red line corresponds to 0.5D and black line to 0.25D. Cell 1 is central cell, cells 2–7 are first neighbors, and cells 8–19 are second neighbors. For a given radionuclide distribution (e.g., intracytoplasmic), same simulation allowed assessment of absorbed doses to cell membranes and to nuclei. CS = cell surface; Cy = cytoplasm; M = membranes; N = nuclei.
With an intracytoplasmic radionuclide location, membrane and nuclear absorbed doses to the 4 unlabeled cells ranged between 0.25D and 0.5D, both for 177Lu and for 161Tb (Fig. 3). The absorbed dose to a given cell also depends on its position and the labeling state of adjacent cells.
With the radionuclide at the cell surface, nonuniform targeting resulted in substantial heterogeneity in absorbed doses to cell membranes (Fig. 3). With 177Lu, unlabeled cells received between 2.3 and 4.5 Gy, or on average only 9.6% of the mean dose for a homogeneously targeted cluster (38.9 Gy). With 161Tb, heterogeneity is even more pronounced. Absorbed doses to membranes of unlabeled cells ranged between 5.0 and 12.4 Gy, or on average only 2.9% of the dose with uniform targeting (238 Gy). The impact on nuclear absorbed doses is here lower. The nuclei of unlabeled cells received on average 60% of the 177Lu absorbed doses, or 48% of the 161Tb doses, as compared with a cluster with uniform targeting (Fig. 3).
With intranuclear 177Lu (Fig. 3), the nuclei of unlabeled cells received 1.7–3.0 Gy, or on average 17.2% of the dose expected with uniform targeting (14.0 Gy). With 161Tb, unlabeled cells received 3.5–5.9 Gy, or only 10.8% of the dose expected with uniform cell targeting (44.0 Gy).
Assessment of Dual Targeting as a Strategy to Compensate for Heterogeneity
With an intracytoplasmic radionuclide, dual targeting minimized heterogeneities in membrane and nuclear absorbed doses (Fig. 4). Most unlabeled cells, which had dose levels between 0.25D and 0.5D, reached 0.5D with dual targeting. Because of the stochastic aspect, 1 cell in the 161Tb simulation was untargeted by either radiopharmaceutical and stayed at about 0.25D. In our model (4/19 untargeted cells), the probabilities that clusters contain one or more cells missed by both radiopharmaceuticals are about 47% for 1 cell, 6.3% for 2 cells, 1.6% for 3 cells, and 0.03% for all 4 cells.
Absorbed doses in situations of nonuniform cell targeting: comparison between single and dual targeting. For single targeting, nonuniform targeting is in blue, with dark blue corresponding to labeled cells and light blue to 4 unlabeled cells. For dual targeting, absorbed doses from first radiopharmaceutical are in blue (dark blue for labeled cells and light blue for unlabeled cells), whereas absorbed doses delivered by second radiopharmaceutical are in red (dark red for labeled cells and light red for unlabeled cells). CS = cell surface; Cy = cytoplasm; M = membranes; N = nuclei.
With the radionuclide at the cell surface, and the membrane as the target, dual targeting showed substantial benefit (Fig. 4). With 177Lu, in 3 cells with a dose initially less than 12%, the mean dose reached 0.5D with the second radiopharmaceutical. With 161Tb, again because of the stochastic aspect, only 2 cells received compensation, moving from 2.2% of the mean dose to 0.5D. As heterogeneities in nuclear absorbed doses were less pronounced, dual targeting had almost no impact (177Lu) or only modest benefit (161Tb) (Fig. 4).
With an intranuclear radionuclide location, dual targeting was beneficial in minimizing heterogeneities in nuclear absorbed doses (Fig. 4). With 161Tb, for example, 3 of the 4 unlabeled cells, with a dose level well below 0.25D, reached 0.5D level at the second targeting. Compensation was accompanied by a decrease in absorbed dose to other cells in the cluster, which, however, remained above the 0.5D level.
DISCUSSION
Used as adjuvant therapy to target CTC and micrometastases, or as consolidation therapy for minimal residual disease, TRT has the potential to be curative (5–9,31). Radionuclides that can increase the absorbed dose in tiny tumors would be relevant in these settings. 161Tb, a β−-emitter with coemissions of Auger electrons, is one interesting candidate (12–17). Interest in 161Tb is growing, and 2 clinical trials on patients with advanced disease have started recruitment. The phase I/II trial VIOLET is assessing the safety and efficacy of 161Tb-PSMA-I&T in men with castration-resistant prostate cancer (NCT05521412). A phase 0 proof-of concept study is measuring the therapeutic index of the somatostatin antagonist 161Tb-DOTA-LM3, in comparison to 177Lu-DOTATOC, in patients with gastroenteropancreatic neuroendocrine tumors (NCT05359146).
In our tumor cluster model, when all 19 cells were targeted, and depending on the location of the radionuclide, 161Tb delivered a 2- to 3-fold higher nuclear absorbed doses than 177Lu but also 2- to 6-fold higher absorbed doses to cell membranes (Tables 1 and 2; Fig. 2). Interaction of ionizing radiation with the cell membrane induces sphingomyelin hydrolysis to ceramide, initiating apoptosis (32). Since a number of radiopharmaceuticals reside on the membrane without being internalized (e.g., neuropeptide antagonist analogs and many antibodies), understanding the role of the cell membrane as a target becomes particularly important, specifically for TRT. Membrane irradiation by Auger electrons or α-particles is highly cytotoxic through various mechanisms (23,24,33). With the radionuclide at the cell surface, absorbed doses to cell membranes were higher than nuclear doses, both with 177Lu (7.4-fold higher: 38–41 vs. 4.7–7.2 Gy) and with 161Tb (22-fold higher: 237–244 vs. 9.8–15.1 Gy) (Tables 1 and 2). Also, 161Tb showed substantial superiority (161Tb/177Lu dose ratio, ∼6.1) (Table 1; Fig. 2). Importantly, a recent preclinical study showed highly enhanced efficacy for TRT with 161Tb-labeled somatostatin antagonists that stay at the cell membrane (34).
Damage to membranes can also impair the motility and invasion abilities of cells (35), which may impact the fate of CTC. Therefore, the impact of radiopharmaceuticals in this regard also deserves investigation.
When aiming to eradicate small tumors, the potential impact of nonuniform cell targeting should be assessed (19–21). Loss of target expression can be present from the outset or occur during disease evolution or under pressure from previous therapies. We modeled a situation of moderate nonuniformity in which 4 of 19 cells were unlabeled, their positions within the cluster being stochastically determined. With an intranuclear radionuclide, nuclear absorbed doses to unlabeled cells were on average only 17.2% (177Lu) or 10.8% (161Tb) those obtained with uniform targeting (Fig. 3), pointing to the importance of the self-dose (Table 2). Thus, efforts toward achieving an intranuclear location for Auger emitters (36,37) should also aim at targeting of all cells. With intracytoplasmic radionuclides, absorbed doses to the membranes and nuclei of unlabeled cells were 25%–50% those obtained with uniform targeting (Fig. 3). With cell surface radiopharmaceuticals, nonuniform targeting resulted in major heterogeneity in absorbed doses to cell membranes but not to nuclei. Membranes of unlabeled cells received about 9.6% of the 177Lu absorbed dose or about 2.9% of the 161Tb dose, compared with uniform targeting (Fig. 3).
Dual targeting is being actively investigated in cancer therapy to counter tumor heterogeneity (25). Multiple targeting is also possible with TRT (1,26,27). If the organs at risk differ, then an appropriate combination of 2 radiopharmaceuticals might also offer better tolerance (1,26). Through Monte Carlo simulation, we assessed whether dual targeting may minimize absorbed dose heterogeneities. With an intranuclear radionuclide location, dual targeting appeared helpful (Fig. 4). Developing many radiopharmaceuticals having an intranuclear location might not be simple, however. With an intracytoplasmic radionuclide, dual targeting showed some benefit (Fig. 4). With cell surface radiopharmaceuticals, dual targeting showed a major benefit in reducing cell membrane dose heterogeneities (Fig. 4), with little impact on nuclear absorbed doses. The benefit from dual targeting would thus depend on the relative importance of the cell membrane as a target (23,24,34). Dual targeting is feasible given the increasing number of identified cell surface targets and designed radioligands.
Our study had some limitations. We considered cells with a uniform size, spheric shape, and centered nucleus. Cell targeting was considered binary (labeled/unlabeled); activity content can be more nuanced. Only one simulation was performed for each situation. Our aim was simply to help understand the relative merit of diverse targeting strategies (Figs. 3 and 4). With dual targeting, we considered 2 radiopharmaceuticals in the same cell compartment, with the same radionuclide. Other approaches, such as combining internalizing and noninternalizing radiopharmaceuticals or different radionuclides, can be envisioned. In this work, we focused on 2 targets: the nucleus and the cell membrane (23,24). However, cytoplasmic organelles, such as mitochondria and lysosomes, can also play a role in inducing cell death from a dose deposit linked to internalizing peptides or antibodies that could have a strong cytotoxic effect when using Auger or α-emitters (33,38). In future work, we intend to also model the dose deposit in cytoplasm and cytoplasmic organelles with CELLDOSE from 177Lu, 161Tb, and Auger emitters. Finally, besides effects on targeted cells, TRT can also impact nontargeted cells through bystander effects or immune responses (33,39,40). Indeed, absorbed dose is only one step toward understanding the complexity of radiobiologic effects in TRT (33,40).
CONCLUSION
When aiming at CTC clusters, micrometastases, or minimal residual disease, 161Tb is a better candidate than 177Lu, delivering higher absorbed doses. The role of the cell membrane as a target deserves attention. With cell surface radiopharmaceuticals, doses to cell membranes are high—notably so with 161Tb. Nonuniform cell targeting leads to absorbed dose heterogeneity that can impact the efficacy of TRT. Dual targeting can minimize this heterogeneity and should be further investigated.
DISCLOSURE
This study was conducted in the framework of the University of Bordeaux IdEx “Investments for the Future” program RRI “NewMOON.” No other potential conflict of interest relevant to this article was reported.
KEY POINTS
QUESTION: Is the novel radionuclide 161Tb suitable for TRT of tumor cell clusters?
PERTINENT FINDINGS: Our Monte Carlo simulations showed that 161Tb delivers higher absorbed doses than 177Lu to nuclei and cell membranes, whatever the location of a radiopharmaceutical. Nonuniform cell targeting resulted in absorbed dose heterogeneity that could be countered through dual targeting.
IMPLICATIONS FOR PATIENT CARE: 161Tb can be a better radionuclide for clinical trials aiming at eradicating tumor cell clusters and micrometastases.
Footnotes
Published online Jun. 15, 2023.
- © 2023 by the Society of Nuclear Medicine and Molecular Imaging.
REFERENCES
- Received for publication January 25, 2023.
- Revision received May 11, 2023.