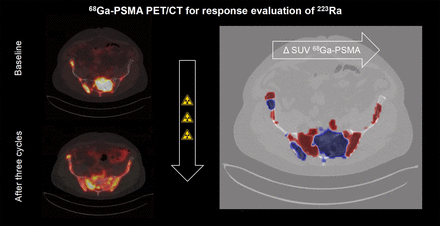
Visual Abstract
Abstract
CT and bone scintigraphy are not useful for response evaluation of bone metastases to 223Ra treatment in metastatic castration-resistant prostate cancer (mCRPC). PET using 68Ga prostate-specific membrane antigen 11 (68Ga-PSMA) is a promising tool for response evaluation of mCRPC. The aim of this study was to determine the utility of 68Ga-PSMA PET/CT for response evaluation of 223Ra treatment in patients with mCRPC. Methods: Within this prospective, multicenter, imaging discovery study, 28 patients with mCRPC, eligible for 223Ra treatment, were included between 2019 and 2022. Patients received 223Ra according to the standard of care. Study procedures included CT, bone scintigraphy, and 68Ga-PSMA PET/CT at baseline, after 3 and 6 cycles of 223Ra treatment, and on treatment failure. Response to 223Ra treatment was visually assessed on all 3 imaging modalities. Total tumor volume within bone (TTVbone) was determined on 68Ga-PSMA PET/CT. Intrapatient heterogeneity in response was studied using a newly developed image-registration tool for sequential images of PET/CT. Results were compared with failure-free survival (good responders vs. poor responders; cutoff, 24 wk) and alkaline phosphatase (ALP) response after 3 cycles. Results: Visual response assessment criteria could not distinguish good responders from poor responders on 68Ga-PSMA PET/CT and bone scintigraphy. For 68Ga-PSMA PET/CT, TTVbone at baseline was lower in good responders than in poor responders, whereas TTVbone increased in both groups during treatment. TTVbone was higher in patients with new extraosseous metastases during 223Ra treatment. Although TTVbone and ALP correlated at baseline, changes in TTVbone and ALP on treatment did not. 68Ga-PSMA response of TTVbone showed intrapatient heterogeneity in most patients. Conclusion: mCRPC patients with lower TTVbone on 68Ga-PSMA PET/CT have the best clinical outcome after 223Ra treatment. Response is highly heterogeneous in most patients. A decrease in ALP, which occurred in most patients, was not correlated with a decrease in TTVbone, which might make one question the value of ALP for disease monitoring during 223Ra treatment in clinical practice.
Because bone metastases, which occur in up to 90% of patients with metastatic prostate cancer, are associated with severe pain and pathologic fractures, effective treatment is needed (1,2). However, the value of diagnostic CT (dCT) and bone scintigraphy is hampered for early response evaluation of bone metastases. On dCT, bone metastases of prostate cancer, which are often osteoblastic, cannot be distinguished from osteosclerosis (3,4). In addition, bone scintigraphy has a low specificity and is prone to flare phenomena. Therefore, confirmation of progressive bone metastases is required on a second bone scintigraphy after the start of treatment according to the Prostate Cancer Working Group 3 criteria (5–7).
PET using 68Ga prostate-specific membrane antigen 11 (68Ga-PSMA) is a useful imaging modality for diagnosis, staging, and response evaluation of prostate cancer (8–11). Correlations between 68Ga-PSMA uptake, prostate-specific antigen, and patient survival have been reported for patients with metastatic castration-resistant prostate cancer (mCRPC) (12–18). In comparison with conventional imaging, at least one advantage of 68Ga-PSMA PET/CT is image acquisition by a single machine.
223Ra, an α-emitting radionuclide, is indicated for treatment of patients with mCRPC and bone metastases without visceral metastases (19,20). However, besides the limited value of dCT and bone scintigraphy for early response evaluation of bone metastases, blood-based biomarkers, including prostate-specific antigen, cannot predict a response to 223Ra. Serum alkaline phosphatase (ALP) is the best biomarker to date but correlates only moderately with patient overall survival after 223Ra treatment (6,21). Therefore, new tools are needed to evaluate bone metastases and to guide clinical decision-making on continuation of 223Ra treatment.
In this prospective imaging and biomarker discovery study, we investigated the use of 68Ga-PSMA PET/CT for response evaluation after 223Ra treatment in patients with mCRPC. Images of 68Ga-PSMA PET/CT were compared with images of conventional modalities, and changes in total tumor volume during 223Ra treatment were analyzed. In addition, we developed a widely applicable image-registration tool to merge sequential PET/CT images and to quantify intrapatient heterogeneity of 68Ga-PSMA uptake to measure the response in bone metastases.
MATERIALS AND METHODS
Study Design
This multicenter study (Radium223Insight, Dutch Trial Register NL7380) included patients at the Erasmus Medical Center Cancer Institute, Franciscus Gasthuis and Vlietland Hospital, and Radboud University Medical Center, The Netherlands. The study was approved by the institutional review boards (MEC 18-1562). Patients received 6 consecutive injections with 223Ra at an interval of 4 wk. In the case of progression of disease, based on the Prostate Cancer Working Group 3 criteria, or severe toxicity, treatment was discontinued. Study procedures consisted of blood draws, tumor tissue biopsies, and imaging, including sequential 68Ga-PSMA PET/CTs, dCT of the thorax and abdomen, and bone scintigraphy (Supplemental Fig. 1; supplemental materials are available at http://jnm.snmjournals.org). All patients provided written informed consent before the start of the study procedures.
Patients
Patients with mCRPC and predominantly bone metastases were eligible if they had progressive disease, received at least 2 prior treatment lines (unless the patient was not able or willing to receive other treatments), and had not received prior chemotherapy other than docetaxel. Detailed inclusion and exclusion criteria are described in the supplemental materials. Because of the explorative nature of the study, the sample size was arbitrarily set at 30 patients. However, because of delayed inclusion due to the coronavirus disease 2019 pandemic, the decision was made to close the trial for further accrual after the inclusion of 28 patients with completed follow-up between February 2019 and January 2022.
Study Endpoints
The primary endpoint was failure-free survival (FFS), defined as the time from the start of 223Ra treatment to the next line of treatment, best supportive care, or death. The next treatment or best supportive care was started on clinical, biochemical, or radiologic signs of progressive disease, according to the Prostate Cancer Working Group 3 criteria (7). A composite endpoint was chosen because a reliable parameter of disease response and progression during 223Ra treatment is lacking. To avoid bias in treatment decisions, treating physicians were unaware of the results of 68Ga-PSMA PET/CT, except for the baseline scan. Detection of visceral metastases or other significant findings was reported by the nuclear medicine physician to the treating physician.
Here, we report on the primary endpoint and parameters based on sequential 68Ga-PSMA PET/CT, dCT, and bone scintigraphy and longitudinal measurements of serum ALP (Supplemental Fig. 1). Other endpoints and parameters will be reported in later publications.
Image Acquisition
68Ga-PSMA PET/CT, dCT, and bone scintigraphy were performed at baseline, after 3 cycles of 223Ra treatment, at the end of the treatment (after 6 cycles of 223Ra treatment), and at treatment failure (Supplemental Fig. 1). Details on image acquisition are described in the supplemental materials (22).
Image Analyses
Longitudinal 68Ga-PSMA PET/CT, dCT of the thorax and abdomen, and bone scintigraphy images were visually assessed. In addition, semiautomatic assessment of total tumor volume within bone (TTVbone) and analyses of heterogeneity in response were performed for 68Ga-PSMA PET/CT. The 3 imaging modalities were mutually compared and correlated with FFS and ALP response. Details are described in the supplemental materials (4,7,23).
Visual Response Evaluation
68Ga-PSMA PET/CT images were examined by nuclear medicine physicians according to adapted PERCIST (24,25). The dCT and bone scintigraphy were assessed according to RECIST version 1.1 and the 2 + 2 rule, respectively (4,7).
Semiautomatic Assessment of TTVbone
TTVbone on 68Ga-PSMA PET images was semiautomatically measured using a PERCIST-based lesion selection tool (Hermes Hybrid3D 3.0.1). In addition to assessing FFS and ALP response, we compared patients with and without new extraosseous metastases during treatment.
Analyses of Heterogeneity in Response
An image-registration tool was developed to merge 2 sequential PET/CT images. The difference in 68Ga-PSMA uptake between baseline and follow-up was calculated per voxel for all previously selected tumor lesions using TTVbone. All voxels within the merged tumor mask of an individual patient were categorized on the basis of changes in 68Ga-PSMA uptake over time, and the intrapatient distribution of the categories was explored.
Statistical Analyses
Patients were categorized as good or poor responders using a cutoff at 24 wk of FFS, which is similar to the period of 6 cycles of 4 weekly injections with 223Ra. Baseline characteristics, clinical outcomes, and parameters of 68Ga-PSMA were described as mean ± SD, median and interquartile range (IQR), or number of events and percentage. Depending on the format and normality distribution of the data, the appropriate statistical tests were used. Applied statistical tests are described in the figure legends. All P values were 2-sided, and a P value of 0.05 or less was considered to be significant. No corrections for multiple testing were performed.
RESULTS
Patient Characteristics and Clinical Outcomes
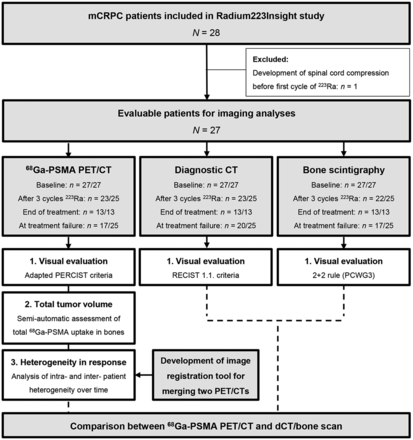
Of the 28 included patients, 27 patients were evaluable (Fig. 1). On the basis of FFS, patients were categorized as good (n = 13) or poor responders (n = 12). Two patients, who discontinued treatment because of hematologic adverse events, were not included in the responder assessments. No significant differences between good and poor responders were found in baseline clinical characteristics. Serum lactate dehydrogenase at baseline was significantly higher in poor responders than in good responders (262 U/L [IQR, 229–330 U/L] vs. 201 U/L [IQR, 186–231 U/L]; P = 0.001), whereas serum prostate-specific antigen and ALP were not different (Supplemental Table 1).
Flowchart of patient inclusion: description of patient inclusion, evaluable scans, and performed analyses. 68Ga-PSMA PET/CT, dCT, and bone scintigraphy were visually assessed according to adapted PERCIST, RECIST version 1.1, and 2 + 2 rule as described in Prostate Cancer Working Group 3 criteria, respectively.
Median FFS was 25.9 wk (IQR, 25.0–35.1 wk) and 11.7 wk (IQR, 10.0–17.3 wk) for good and poor responders, respectively (P < 0.001). Four of 13 (31%) good responders developed extraosseous disease during treatment, whereas this occurred in 8 of 12 (67%) poor responders (P = 0.068). Good responders had a longer median overall survival than poor responders (91.0 wk [IQR, 67.1–102.3 wk] vs. 27.0 wk [IQR, 16.4–48.0 wk], P = 0.004). Dynamics in prostate-specific antigen and ALP were not different between the 2 groups (Table 1).
Clinical Outcomes of Included Patients
Visual Response Evaluation of 68Ga-PSMA PET/CT, dCT, and Bone Scintigraphy
All scans were visually assessed (Fig. 1; Supplemental Table 2). Of the 21 patients who underwent all 3 imaging modalities, 17 (81%) and 4 (19%) patients had progressive disease and stable disease on 68Ga-PSMA PET/CT (PERCIST) (24), respectively, whereas the combination of dCT (RECIST version 1.1) (4) and bone scintigraphy (2 + 2 rule) (7) resulted in progressive disease, nonprogressive disease, and nonevaluable disease in 2 (10%), 10 (48%), and 9 (43%) of 21 patients after 3 cycles of 223Ra treatment, respectively. Two patients with progressive disease on conventional imaging also had progressive disease on 68Ga-PSMA PET/CT, whereas 15 patients with progressive disease on 68Ga-PSMA PET/CT had nonprogressive disease or were not evaluable on conventional imaging (Fig. 2). At the end of treatment and at treatment failure, all patients had progressive disease on 68Ga-PSMA PET/CT, whereas 15% and 43% of patients, respectively, also had progressive disease on conventional imaging (Supplemental Fig. 2).
Visual response evaluation after 3 cycles of 223Ra treatment. 68Ga-PSMA PET/CT, dCT, and bone scintigraphy were visually assessed according to adapted PERCIST, RECIST version 1.1, and 2 + 2 rule as described in Prostate Cancer Working Group 3 criteria. Visual response evaluation results are after 3 cycles of 223Ra treatment for patients who were evaluable for all 3 imaging modalities (n = 21). NE = nonevaluable disease; PD = progressive disease; SD = stable disease.
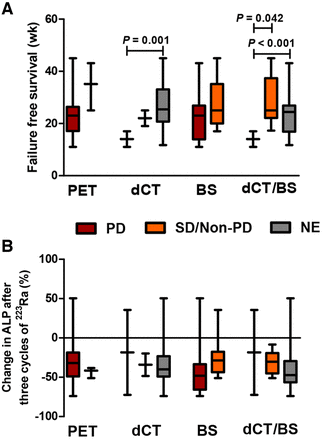
For those patients who discontinued treatment because of progressive disease and underwent all 3 imaging modalities (n = 20), FFS was compared between the response categories after 3 cycles of 223Ra treatment of each imaging modality. For 68Ga-PSMA PET/CT, the median FFS for patients with progressive disease and stable disease was 23 wk (IQR, 17–26 wk; n = 17) and 35 wk (IQR, 25–43 wk; n = 3), respectively (P = 0.362). For dCT, the median FFS was higher in patients with nonevaluable disease than in patients with progressive disease (25 wk [IQR, 20–27 wk; n = 16] vs. 11 wk [IQR, 11–17 wk; n = 2]; P = 0.001), whereas FFS was comparable in patients with stable disease (19 wk [IQR, 19–25 wk; n = 2]; P = 0.090). For bone scintigraphy, the median FFS was comparable for at least 2 or more new lesions and fewer than 2 new lesions (23 wk [IQR, 16–27 wk; n = 9] vs. 25 wk [IQR, 20–35 wk; n = 11]; P = 0.396; Fig. 3A). The median change in ALP after 3 cycles of 223Ra treatment was not different between the response categories for any of the 3 imaging modalities (Fig. 3B).
Visual response evaluation after 3 cycles of 223Ra treatment in relation to FFS and ALP response. (A) FFS in response evaluation categories of 3 imaging modalities. All patients who underwent all 3 imaging modalities and discontinued treatment because of progression of disease (and not because of toxicity) were included (n = 20). FFS was compared between response categories within each imaging modality using log-rank test. (B) ALP response after 3 cycles of 223Ra treatment (n = 20) was compared between response categories within each imaging modality using Kruskal–Wallis test. PD = progressive disease; SD = stable disease; NE = nonevaluable disease; PET = 68Ga-PSMA PET/CT; BS = bone scan.
Semiautomatic Assessment of TTVbone on 68Ga-PSMA PET/CT
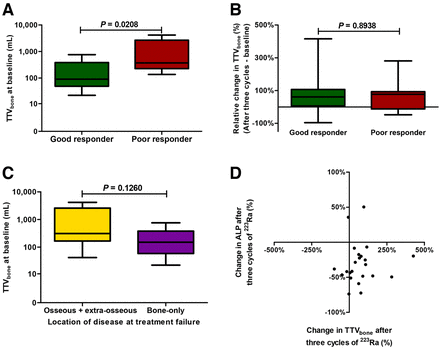
At baseline and after 3 cycles of 223Ra treatment, the good responders had lower median TTVbone than the poor responders: 90 cm3 (IQR, 48–385 cm3; n = 13) versus 372 cm3 (IQR, 227–2,664 cm3; n = 12) (P = 0.0208) and 161 cm3 (IQR, 84–515 cm3; n = 13) versus 926 cm3 (IQR, 405–2,941 cm3; n = 9) (P = 0.0384), respectively (Fig. 4A; Supplemental Fig. 3A). For good and poor responders, the median absolute change in TTVbone after 3 cycles of 223Ra treatment was +56 cm3 (IQR, 12–237 cm3; n = 13) and +348 cm3 (IQR, −45 to +817 cm3; n = 9) (P = 0.3853), whereas the median relative changes were +61% (IQR, +7% to +107%; n = 13) and +77% (IQR, −12% to +93%; n = 9) (P = 0.8938), respectively (Fig. 4B; Supplemental Fig. 3B). Three poor responders did not undergo 68Ga-PSMA PET/CT after 3 cycles of 223Ra treatment because of earlier treatment failure (n = 2) and patient withdrawal (n = 1). TTVbone at other time points is shown in Supplemental Figures 3A–3F.
TTVbone on 68Ga-PSMA PET/CT. (A) Absolute TTVbone in good responders (n = 13) and poor responders (n = 12) at baseline. (B) Relative change in TTVbone from baseline to after 3 cycles of 223Ra treatment in good (n = 13) and poor responders (n = 9). (C) TTVbone in patients with and without new extraosseous metastases during 223Ra treatment at baseline (n = 12 and 12, respectively; 1 not specified because of absence of imaging at time of treatment failure). Groups were compared using Mann–Whitney U test. (D) Relative change in ALP and TTVbone after 3 cycles of 223Ra treatment did not correlate (Spearman correlation coefficient, 0.1739; n = 23; P = 0.4274).
At the time of treatment failure, TTVbone was higher in patients with extraosseous disease than in patients without extraosseous disease during treatment with 223Ra (median, 1,835 cm3 [IQR, 466–2,948 cm3; n = 7] vs. 308 cm3 [IQR, 150–697 cm3; n = 9]; P = 0.0115; Supplemental Fig. 3H). This association was also seen at baseline (median TTVbone, 311 cm3 [IQR, 167–2,572 cm3; n = 12] vs. 151 cm3 [IQR, 59–380 cm3; n = 12]; P = 0.1206) and after 3 cycles of 223Ra treatment (median TTVbone, 926 cm3 [IQR, 182–2,823 cm3; n = 9] vs. 159 cm3 [IQR, 72–624 cm3; n = 12]; P = 0.0302; Fig. 4C; Supplemental Figs. 3G and 3H).
TTVbone and ALP were positively correlated at baseline and after 3 cycles, with Spearman correlation coefficients of 0.5413 (n = 27; P = 0.0035) and 0.6500 (n = 23; P = 0.0008), respectively (Supplemental Figs. 3I and 3J). Nevertheless, after 3 cycles of 223Ra treatment, most patients showed a decrease in ALP, whereas TTVbone increased, resulting in a Spearman correlation coefficient of 0.1739 (n = 23; P = 0.4274; Fig. 4D).
Heterogeneity on 68Ga-PSMA PET/CT in Response to 223Ra Treatment
During visual response evaluation, mixed responses in individual patients were observed. Therefore, we developed an image-registration tool that merges 2 sequential images of 68Ga-PSMA PET/CT to visualize and quantify heterogeneity in response over time (Fig. 5; Supplemental Figs. 4 and 5; Supplemental Video 1).
In-house–developed image-registration tool for visualization and quantification of heterogeneity in tumor response. Image registration to merge 2 sequential images of 68Ga-PSMA PET/CT consists of 4 steps. (A) Both scans are cropped to comparable field of view, and bed is removed from images. (B) Bone masks are obtained by region-growing algorithm with threshold of 150 Hounsfield units on low-dose CT. (C) Initial alignment of both images is performed by rigid-body registration. (D) To correct for differences in patient posture between scans, deformable B-spline registration is applied using isotropic mesh size of ∼10-cm distance between nodes. (E) Transformations are subsequently applied to associated PET images and tumor mask that were obtained during TTVbone assessment. Changes in SUVs of 68Ga-PSMA are color-scaled, showing increasing (i.e., red color) and decreasing (i.e., blue color) 68Ga-PSMA uptake over time. HU = Hounsfield units.
After 3 cycles of 223Ra treatment, 68Ga-PSMA uptake decreased, stabilized, and increased with a median of 32% (IQR, 18%–40%), 21% (IQR, 12%–26%), and 53% (IQR, 29%–65%), respectively, of TTVbone (n = 21; Fig. 6; Supplemental Fig. 4A). A higher fraction of decreased 68Ga-PSMA uptake was correlated with a higher decrease in TTVbone after 3 cycles of 223Ra treatment (Spearman correlation coefficient, −0.8156; n = 21; P < 0.0001; Supplemental Fig. 4G). At the time of treatment failure compared with after 3 cycles of 223Ra treatment, the fraction of progressive 68Ga-PSMA uptake increased from 53% (IQR, 28%–67%) to 78% (IQR, 56%–84%; n = 13) (P = 0.001; Supplemental Fig. 4E).
Quantification of heterogeneity in tumor response on 68Ga-PSMA PET/CT after 3 cycles of 223Ra treatment. Changes in SUV of 68Ga-PSMA are categorized for every voxel within TTVbone according to decreased 68Ga-PSMA uptake (baseline to follow-up SUV ≤ −30%), stable 68Ga-PSMA uptake (baseline to follow-up SUV = −30% to +30%), and increased 68Ga-PSMA uptake (baseline to follow-up SUV ≥ 30%) and visualized for good and poor responders after 3 cycles of 223Ra treatment (n = 13 and 8). G = good responder; P = poor responder.
The fraction of decreased 68Ga-PSMA uptake after 3 cycles of 223Ra treatment did not correlate with TTVbone at baseline (Spearman correlation coefficient, −0.04880; n = 21; P = 0.8336) and was comparable between good and poor responders (median, 32% [IQR, 14%–40%; n = 13] vs. 29% [IQR, 18%–55%; n = 8]; P = 0.547; Fig. 6; Supplemental Fig. 4F). However, the fraction of decreased 68Ga-PSMA uptake and change in ALP after 3 cycles of 223Ra treatment showed a significant correlation (Spearman correlation coefficient, −0.4580; n = 21; P = 0.0368; Supplemental Fig 4H).
DISCUSSION
In this prospective multicenter study, we investigated the value of 68Ga-PSMA PET/CT to evaluate mCRPC during treatment with 223Ra.
To compare 68Ga-PSMA PET/CT with conventional techniques, such as dCT and bone scintigraphy, we visually assessed all 3 imaging modalities, using standardized evaluation criteria. After 3 cycles of 223Ra treatment, bone scintigraphy could not distinguish good responders from poor responders to 223Ra treatment. In addition, many patients were nonevaluable on the basis of conventional imaging, because at least 2 new lesions on bone scintigraphy needed confirmation on a second scan according to the 2 + 2 rule, and dCT is not suitable for response evaluation of bone-only disease. 68Ga-PSMA PET/CT has an increased diagnostic accuracy and the advantage of tomography in comparison with planar bone scintigraphy (11). However, when PERCIST was used for 68Ga-PSMA PET/CT, most patients had progressive disease due to the development of at least 1 new bone lesion. In addition, we observed intrapatient heterogeneity in response, which was not reflected by PERCIST. Therefore, PERCIST was not considered sufficient to distinguish good responders from poor responders to 223Ra treatment, and we decided to assess novel parameters of 68Ga-PSMA PET/CT.
Using semiautomatic assessment of tumor volume on 68Ga-PSMA PET/CT, we found that good responders had a lower TTVbone than poor responders at baseline and after 3 cycles of 223Ra treatment. However, since a baseline tumor load is associated with the prognosis of patients with mCRPC in general, this finding might not be specific for 223Ra treatment (26). Nevertheless, higher TTVbone was associated with new extraosseous disease during treatment, which might be considered at the start of 223Ra treatment. Although improved clinical outcome is, in general, associated with radiologic response, a comparable increase in TTVbone after 3 cycles of 223Ra treatment was observed in both good and poor responders. This might be caused by the arbitrary cutoff for responders at 24 wk of FFS and by confounding factors such as baseline tumor load and the development of extraosseous metastases. Therefore, validation of the association between TTVbone and clinical outcome, including the correction of confounding factors, in a larger patient cohort is required to further clarify the value of 68Ga-PSMA PET/CT for response evaluation during treatment with 223Ra.
Remarkably, a decrease in TTVbone was not associated with a decrease in ALP, whereas absolute ALP values did correlate with TTVbone. This might be caused by the fact that ALP reflects the activity of osteoblasts, which are targeted by 223Ra, but does not directly reflect the tumor load. In the ALSYMPCA trial, it was shown that ALP dynamics during treatment with 223Ra correlate with the risk of death but cannot be used as a surrogate for overall survival (21). Thus, in clinical practice, an ALP decrease after 223Ra treatment is not necessarily associated with tumor response on 68Ga-PSMA PET/CT and could coexist with radiologic disease progression.
Using our in-house–developed image-registration tool, we gained more insight into the unexpected changes in TTVbone. Remarkably, most patients had a significant intrapatient heterogeneity, showing a typical pattern of decreased 68Ga-PSMA uptake in the original region of the bone metastasis and increased 68Ga-PSMA uptake in the surrounding bone tissue after 223Ra treatment (Supplemental Fig. 5). Although the distinct dynamics in 68Ga-PSMA uptake over time suggest a change in tumor load and location, the upregulating effect of irradiation on PSMA expression in tumor cells should be considered as a potential factor in measuring tumor volume on 68Ga-PSMA PET/CT (27). In addition, PSMA is expressed not only on prostate cancer cells but also on the neovasculature of several solid tumors, including prostate cancer (28,29). Because radiation can induce angiogenesis, the increased PSMA expression in the surrounding bone tissue might also be the result of neovascularization in response to treatment with 223Ra (30,31).
On the basis of our results, we hypothesized that the correlation between ALP and TTVbone disappears because of the interruption of the osteoblast–tumor interaction by 223Ra, as explained in Supplemental Figure 6 (32). This hypothesis is further supported by the known increase in ALP after discontinuation of 223Ra treatment, suggesting the recovery of the activating tumor–osteoblast interaction (32). In addition, the typical shift of 68Ga-PSMA uptake to the borders of the original tumor lesion on treatment with 223Ra might suggest that the irradiated osteoblasts are no longer a suitable tumor microenvironment, whereas the adjacent undamaged bone tissue still is. Application of the image-registration tool in patients who received other systemic therapies could help to further improve our understanding of heterogeneity in response evaluation in bone metastases.
Other strengths of this study are the prospective design, the extensive follow-up with in-depth imaging, and the masking of 68Ga-PSMA PET/CT to prevent bias on the clinical outcome. Response evaluation of bone metastases was complicated by the clinical endpoint, as extraosseous disease also determines FFS, whereas this is not targeted by 223Ra. Nevertheless, other reliable endpoints directly related to bone metastases and 223Ra are lacking. Therefore, we still consider a clinical endpoint, such as FFS, as the most relevant outcome for 223Ra treatment in current clinical practice.
CONCLUSION
68Ga-PSMA PET/CT could be a useful all-in-one imaging modality for response prediction in patients with mCRPC and predominantly bone disease during treatment with 223Ra. Patients with a lower TTVbone on 68Ga-PSMA PET/CT appear to have the best clinical outcome and lowest chance of developing new extraosseous metastases during treatment. Response to 223Ra shows intra- and intertumor heterogeneity in almost all patients. Remarkably, a decrease in ALP, which occurred in most patients, was not correlated with a decrease in TTVbone, which might make one question the value of ALP for disease monitoring during 223Ra treatment in clinical practice.
DISCLOSURE
This research was financially supported by an unrestricted grant from Bayer and the Foundation Dutch Uro-Oncology Study Group (DUOS) and by the fundraising action Running Stairs for Cancer. Niven Mehra reports institutional grants from Roche, Astrazeneca, MSD, Astellas, BMS, and Pfizer and personal fees from Astrazeneca, MSD, Janssen, Bayer, and Pfizer, all outside the submitted work. Ronald de Wit has speaker roles for Sanofi and Astellas and advisory roles for Merck and Sanofi and received institutional research grants from Sanofi and Bayer. Astrid van der Veldt has received consultancy fees (all paid to the institute) from BMS, MSD, Pierre Fabre, Roche, Pfizer, Sanofi, Novartis, Eisai, and Ipsen. No other potential conflict of interest relevant to this article was reported.
KEY POINTS
QUESTION: Can 68Ga-PSMA PET/CT be used for response evaluation of 223Ra treatment in patients with mCRPC?
PERTINENT FINDINGS: Within this prospective imaging study, response to 223Ra treatment showed high intrapatient heterogeneity on 68Ga-PSMA PET/CT, though patients with a lower TTVbone had the best clinical outcome. Remarkably, changes in TTVbone and ALP were not correlated.
IMPLICATIONS FOR PATIENT CARE: 68Ga-PSMA PET/CT is a useful all-in-one imaging tool for response prediction of 223Ra treatment in patients with mCRPC.
ACKNOWLEDGMENTS
We thank Fred Guurink for organizing the fundraising action Running Stairs for Cancer. Additionally, we acknowledge Stefan Klein for sharing his expertise on image-registration tools and Daniela Oprea-Lager and Elisabeth de Vries for their advice on the manuscript.
Footnotes
Published online Aug. 3, 2023.
- © 2023 by the Society of Nuclear Medicine and Molecular Imaging.
REFERENCES
- Received for publication January 19, 2023.
- Revision received May 31, 2023.