Abstract
2910
Introduction: VMT-α-NET is a modified somatostatin receptor targeted peptide with improved pharmacokinetics and chelation of 203Pb/212Pb. The VMT chelator efficiently chelates 212Pb as well as its daughter 212Bi. Short half-lives of 212Bi daughters 208Tl and 212Po (3 min and 0.3 µs respectively) and strong chelation properties of the VMT-α-NET ensures that α-particles in the decay series are mainly emitted in the target tissue. Together with the γ-emitting 203Pb, ideal for dosimetry, the two radionuclides form an ideal theranostics pair of radionuclides.The goal of this study was to evaluate the labeling procedure for a radiopharmaceutical production of [203Pb]VMT-α-NET for clinical use.
Methods: Hydrochloric acid was purchased from Supelco. Sodium acetate was purchased from Fluka. All other chemical reagents and materials were the highest grade available from Sigma Aldrich, VWR or Acros Organics and used as received without further purification (unless otherwise described). Sep-Pak C18 plus light cartridges were purchased from Waters™. VMT-α-NET and the 50 mg Pb-resin™ cartridges were provided by Viewpoint Molecular Targeting, Inc. (Iowa, USA). Pb-resin™ (100–150 μm) is available commercially from Eichrom Technologies LLC (Lisle, IL USA). 203Pb was purchased from Lantheus Medical Imaging (North Billerica, MA USA) in approximately 1 mL of 0.5 M HCl. The radiochemical purity and the amount of unbound 203Pb in the final product was determined by radio-HPLC using the following conditions: Phenomenex Gemini® 5 µm C18 110 Å LC column 250 x 4.6 mm; flow rate 1 mL/min; solvent A water (0.1% TFA); solvent B acetonitrile (0.1% TFA); gradient from 0 % A to 50 % B within 5 min, 3 min 50% B, gradient from 50 % A to 90 % B after another 3 min, 90 % B for 4 min.
The labeling of VMT-α-NET with 203Pb (4 different synthesis runs) was carried out using a manual procedure as described in detail in [1]. An aliquot of the 203Pb2+ solution (388 – 457 MBq, app. 0.2 mL) in 0.5 M HCl was transferred to the Pb-resin cartridge, which was previously conditioned with 1 mL 0.5 M HCl. The resin was washed using 1 mL of 0.5 M HCl and the 203Pb was subsequently eluted using 2 mL of 0.5 M sodium acetate buffer (pH = 6) into the peptide solution containing a mixture of 50 µg of VMT-α-NET, 296 µL of 0.5 M sodium acetate buffer (pH = 4), 20 µL of a sodium ascorbate solution (50 mg/ml) and 100 µL ethanol (EtOH).The reaction mixture was subsequently heated to 75°C. After 30 minutes the mixture was cooled at room temperature and then transferred to a C-18-SPE cartridge (preconditioned with 1 mL of a solution of 0.5 mL EtOH in 0.5 mL saline (sterile 0.9 % NaCl solution) followed by a washing step using 3 mL saline wherein the labeled peptide is retained on the C-18-cartridge. The cartridge was washed with 1 mL of saline. The 203Pb-labeled VMT-α-NET was finally eluted with 1 mL of a solution of EtOH/saline 50/50 v/v and subsequently rinsed with 9 mL of EtOH/saline 50/50 v/v and subsequently rinsed with 9 mL of a solution of 180 µL sodium ascorbate solution (50 mg/mL) in 9 mL saline. The collected fractions were subsequently passed through a 0.22 µm sterile filter sterile into the final sterile product vial. Samples were taken for quality control analyses (radiochemical purity, chemical purity, pH, radionuclear identity, radionuclear purity, bacterial endotoxin and sterility).
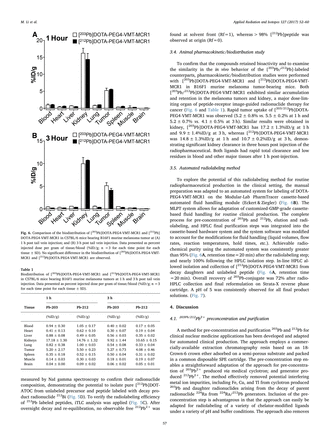
Results: The recovery of 203Pb after the pre-concentration step using Pb-Resin was always > 95%. The RCY ranged from 64 to 79% (after sterile filtration). The radiochemical purity of the product solution was always >99%, with negligible unbound 203Pb2+ (Fig. 1). The pH of the final product was 6. No indication of radiolysis was observed at 1 hour post synthesis. For patient application the product was immediately used after quality control.
Conclusions: A valid radiolabeling procedure for the production of [203Pb]VMT-α-NET for patient application including quality control procedure was established.