Abstract
2504
Introduction: Parkinson’s disease (PD) has been studied using various biomarkers for neurodegeneration. Lewy bodies (LB) in anterior cingulate play a significant role in PD. LB formation includes misfolded, aggregated α-synuclein, membranous organelles, mitochondria damage and other cellular components leading to synaptic dysfunction and cell death. Electron micrographs have shown the presence of mitochondria surrounding the LB. Mitochondria present in the neuron contain monoamine oxidase-A (MAO-A), an enzyme that degrades monoaminergic neurotransmitters. Noninvasive imaging approaches for LB are currently not available. The goal in this study was to evaluate LB using anti-ubiquitin immunohistochemistry (UIHC) and correlate them to MAO-A expression using the PET agent, [18F]FAZIN3. Additionally, [18F]FEPPA was used as a marker for translocator protein (TSPO). Noninvasive PET Imaging agents for LB or surrogate markers for LB may assist in earlier and accurate diagnosis of PD and LB dementia.
Methods: Well-characterized human post-mortem brain tissues consisting of grey matter (GM) anterior cingulate (AC) and white matter (WM) corpus callosum (CC) were obtained from Banner Health Research Institute. Brain samples from controls subjects (CN), n=6; age 81-90 LB=0 and PD, n=6, age 77-89, LB=III-IV were sectioned in a cryotome to provide 10 μm thick slices. Brain slices were immunostained with anti-ubiquitin for LB (UIHC). The UIHC images were analyzed using QuPath for percent anti-ubiquitin in each brain slice as well as LB per unit area (μm2) in the GM cortical layers I-III, IV-VI and WM regions. Adjacent brain slices were incubated [18F]FAZIN3 (1 μCi/cc) in PBS (pH 7.4) buffer at 25 oC for 60 minutes. Using the Optiquant software, regions of interest were drawn in cortical layers I-III, IV-VI and white matter regions and digital light units/mm2 (DLU/mm2) were used to quantify the percentage binding of [18F]FAZIN3. Autoradiographic binding of [18F]FEPPA was carried out similarly. UIHC was correlated with [18F]FAZIN3 and [18F]FEPPA binding.
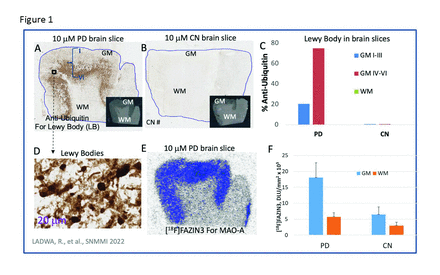
Results: All PD brain samples were positively UIHC stained and confirmed presence of LB. Figure 1A shows the PD AC (inset shows brain slice with GM and WM) with outer cortical layers (I-III) having 21% UIHC while inner layers (IV-VI) had >75% UIHC (Fig-1C). LB were absent in the CN brain slice as expected and all CN subjects grey matter (GM) and whitematter (WM) had <1% UIHC (Fig-1B). Presence of LB was ascertained by closer inspection of UIHC images (Fig-1D), with diameter range of 6-9 μm. Increased [18F]FAZIN3 binding of MAO-A in the AC was observed in all PD subjects (Fig-1E,F). Compared to the CN subjects [18F]FAZIN3 ratio in PD was GM/WM=3.57 while CN subjects was GM/WM=2.24 (Fig-1F). In contrast, [18F]FEPPA binding in the GM and WM was not significantly different between PD and CN subjects. Correlation of UIHC/μm2 (or LB/μm2) with [18F]FAZIN3 binding to MAO-A in DLU/mm2 is shown in Fig-2. There is an increase in [18F]FAZIN3 binding as the UIHC/μm2 increases with a plateauing effect of approx. 1 million LB/mm2. The inset in Fig-2 shows the schematic of a LB, outer layer of which is surrounded by mitochondria, containing MAO-A (in the outer layers). These mitochondria are labeled by [18F]FAZIN3 and because of their localized higher concentration in PD (compared to CN subjects), higher MAO-A binding by [18F]FAZIN3 is observed.
Conclusions: Our results suggest that MAO-A imaging is a good surrogate biomarker for LB. Several PET imaging probes have been used for PD that measure loss of monoaminergic targets and are thus “cold spot” imaging agents. Increased [18F]FAZIN3 binding in PD with the presence of LB is a novel “hot spot” imaging approach. Our results suggests that increased levels of MAO-A in LB due to increased mitochondria in LB may be a sensitive tool in earlier diagnosis of PD. The value of MAO-A imaging in LB may be extended to other neurodegenerative conditions such as LB dementia.