Abstract
2456
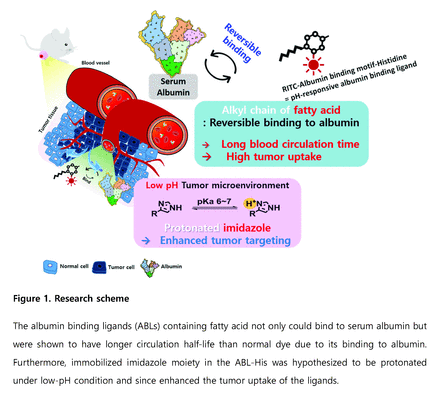
Introduction: Albumin is the most abundant protein in human plasma, has a long circulation half-life (19 days), and transports various molecules through the circulatory system. Therefore, the circulation time and tissue targeting ability of a drug can be enhanced by adding albumin binding motif to the drug. Herein, we developed the pH-responsive albumin-binding ligand that is consist of fatty acid-based albumin binding motif and an imidazole moiety. The imidazole moiety can be protonated at low pH conditions, resulting in reduced interaction with albumin. We hypothesized that our pH-responsive albumin-binding ligand has enhanced tumor targeting ability due to its ability to 1) bind serum albumin and 2) be released at low pH conditions in tumor tissues (Figure 1).
Methods: Albumin-binding ligand (ABL) was synthesized through two major steps as follows: Medium-chain fatty acid, decanoic acid was conjugated to the linker which consist of amide coupling with histidine including an imidazole moiety which is a pH-responsive molecule. Glycine modifed linker was prepared as a control to verify the protonation effects of imidazole moiety. Second, Rhodamine B isothiocyanate (RITC) was substituted with thiourea to the amine part on histidine or glycine for fluorescence imaging (RITC-ABL-His and RITC-ABL-Gly). Desired product was confirmed by nuclear magnetic resonance (NMR), liquid chromatography–mass spectrometry (LC-MS) and then purified them by high performance liquid chromatography (HPLC). Albumin binding and pH-sensitivity studies were conducted by incubating with bovine serum albumin (BSA) solution. In vitro fluorescence imaging was performed by confocal microscopy to confirm cellular uptake. To observe the biodistribution, in vivo imaging system (IVIS) was used. Sort of ligands were intravenously injected tumor-bearing mice for the in vivo fluorescence imaging (free RITC, RITC-ABL-His, and RITC-ABL-Gly). Ex vivo fluorescence images were acquired after resection of major organs. Quantitative analysis was carried out by living image software (n=3).
Results: Synthesized RITC-ABL-His and RITC-ABL-Gly exhibited similar optical properties in absorption and emission spectra. RITC-ABM-His and RITC-ABM-Gly were found to have more than two-fold higher albumin binding capacity (48.3%, 45.6% respectively) compared to free RITC (20.2%) (Figure 2A). RITC-ABM-His showed reduced albumin-binding capacity at low pH conditions (pH 6.5 and 5.5), whereas RITC-ABM-Gly was not altered (Figure 2B), confirming the pH-responsive albumin-binding capacity of RITC-ABM-His. We confirmed that both types of ABL were well uptaken by CT26 colon cancer cells at pH 7.4 using confocal fluorescence microscopy. In vivo and ex vivo fluorescence images were obtained after intravenous injection of RITC-ABM-His, RITC-ABM-Gly and RITC in CT26 tumor bearing-mice. Among the three groups, tumor uptake was the most prominent in RITC-ABM-His injected group (Figure 3A). In addition, the quantified fluorescence intensity of tumors was significantly higher in the RITC-ABM-His group compared to the other two groups (Figure 3B). (P < 0.01, P < 0.01, respectively). These results indicate that RITC-ABL-His can be used as a tumor-specific imaging agent due to its pH-responsive albumin-binding capacity.
Conclusions: We developed a RITC labeled pH-responsive albumin-binding ligand as a cancer imaging agent. The RITC binding site of the pH-responsive albumin-binding ligand can be used as a binding site for anticancer agents, including radionuclides and photosensitizers. Therefore, we anticipate that pH-responsive albumin-binding ligands will be effective anticancer drug platforms based on their extended circulation and pH-responsive tumor-targeting ability.