Visual Abstract
Abstract
In neuroendocrine neoplasms (NENs), the presence of distant metastases has a severe impact on survival leading to a relevant decrease in the 5-y survival rate. Here, 90Y radioembolization (90Y RE) might be an important treatment option; however, data to support clinical benefits for 90Y RE are scarce. Therefore, the purpose of this study was to analyze the use of 90Y RE in NEN patients with hepatic metastases in an international, multicenter retrospective analysis and assess the potential role of 90Y RE in a multimodal treatment concept. Methods: In total, 297 angiographic evaluations in NEN patients before 90Y RE were analyzed. Baseline characteristics and parameters derived from imaging evaluation and 90Y RE were analyzed. Tumor response was assessed using RECIST 1.1, and survival data were collected. Mean overall survival (OS) between different groups was compared using Kaplan–Meier curves and the log rank test. A P value of less than 0.05 indicated statistical significance. Results: After 90Y RE, the disease control rate according to RECIST 1.1 was 83.5% after 3 mo and 50.9% after 12 mo. OS in the entire population was 38.9 ± 33.0 mo. High tumor grade (P < 0.006) and high tumor burden (P = 0.001) were both associated with a significant decrease in OS. The presence of extrahepatic metastases (P = 0.335) and the type of metastatic vascularization pattern (P = 0.460) had no influence on OS. Patients who received 90Y RE as second-line therapy had a slightly longer but not statistically significant OS than patients who had 90Y RE in a salvage setting (44.8 vs. 30.6 mo, P = 0.078). Hepatic and global progression-free survival after 90Y RE was significantly decreased in heavily pretreated patients, compared with patients with second-line therapy (P = 0.011 and P = 0.010, respectively). Conclusion: 90Y RE could be an important alternative to peptide receptor radionuclide therapy as second-line treatment in patients with progressive liver-dominant disease pretreated with somatostatin analogs.
- radionuclide therapy
- neuroendocrine neoplasm
- radioembolization
- SIRT
- neuroendocrine carcinoma
- neuroendocrine tumor
Neuroendocrine neoplasms (NENs) are rare, mostly slow-growing malignancies with an age-adjusted incidence rate of 6.98 per 100,000 in the United States (1). Because of the slow growth of well-differentiated tumors, overall outcomes are favorable, with a 5-y survival rate of up to 82%, depending on the tumor’s primary location, grade according to World Health Organization criteria, and Ki-67 (1–4). As symptoms frequently occur in the later stages of the disease, distant metastases are present in up to 29% of all patients at the time of diagnosis (4). The presence of distant metastases has a severe impact on survival leading to a decrease in the 5-y survival rate from 82% to 35% in well- and moderately differentiated neuroendocrine tumors (NETs) (4). Distant metastases are located predominantly in the liver (2), and complete metastatic resection is possible in approximately only 20% of patients (5). In most cases, somatostatin analog treatment is considered the first-line treatment because of the favorable safety profile and the available evidence (6–8). If hepatic metastases progress during treatment, different therapies including tumor ablation, angiographic procedures such as bland transarterial embolization (TAE) or transarterial chemoembolization (TACE), liver transplantation, and systemic treatments such as chemotherapy or peptide receptor radionuclide therapy (PRRT) are proposed in the guidelines to address different clinical scenarios (9–11).
As hepatic metastases are frequently hypervascularized, injection of an embolic agent into a hepatic artery leads to intrametastatic accumulation of the injected material and consequent necrosis. 90Y radioembolization (90Y RE) might be an important adjunct to these procedures. In contrast to TAE or TACE, all patients have to undergo pretherapeutic angiographic evaluation, including local injection of 99mTc-labeled macroaggregated albumin (99mTc-MAA), subsequent planar scintigraphy, and SPECT or SPECT/CT to exclude relevant extrahepatic shunting. In a second session, intraarterial injection of a calculated dose of 90Y glass microspheres (TheraSphere; Boston Scientific) or resin microspheres (SIR-Spheres; Sirtex Medical) is performed on eligible patients for whole-liver, lobar, or segmental treatment. As hepatic metastases of NETs are usually hypervascularized, these microspheres emit a high local radiation dose to each metastasis. In combination with the short range of the β−-radiation emitted by 90Y, effective local tumor control can be ensured with low systemic toxicity (12,13). Moreover, repeated therapies, as in the case of TAE or TACE, are not necessary. However, data to support clinical benefits for 90Y RE are scarce, as previously published, retrospective analyses suffer from the low number of participants (14–17) or a lack of essential baseline characteristics due to a more technical focus (17,18).
Therefore, our study aimed to provide a retrospective analysis on the current use of 90Y RE in different clinical scenarios and to assess why 90Y RE was not performed on eligible patients to provide data missing from current publications.
MATERIALS AND METHODS
Data Collection
A retrospective data collection of patients with NETs who had undergone angiographic evaluations, including local 99mTc-MAA injection for 90Y RE of hepatic metastases between May 2007 and August 2019, was performed in 6 tertiary-care centers in Europe (Germany and The Netherlands) and 1 in America.
All data were collected using a unified data collection form consisting of these subsections: baseline characteristics; imaging evaluation, including 99mTc-MAA injection; 90Y RE; follow-up examinations after 3 and 12 mo, including tumor response (complete response, partial response, stable disease, and progressive disease) assessed at the contributing center based on RECIST 1.1 (19); and overall survival (OS). Additionally, hepatic and global progression-free survival (PFS) was assessed according to RECIST 1.1 (Supplement 1; supplemental materials are available at http://jnm.snmjournals.org).
Statistical Analysis
For all patients after undergoing the first 90Y RE, survival characteristics were investigated using Kaplan–Meier curves and the log rank test for tumor grade, hepatic tumor burden, tumor vascularization, the presence of extrahepatic metastases, and the type of microspheres used for 90Y RE. In addition, Kaplan–Meier curves and the log rank test were used to analyze differences in OS, as well as hepatic and global PFS, between patients who had undergone only surgery for the primary tumor or metastases and somatostatin analog treatment before 90Y RE (second-line 90Y RE) and patients with extensive systemic pretreatment (therapy additional to surgery for the primary tumor or metastases and somatostatin analog treatment before 90Y RE [salvage 90Y RE]).
A P value of less than 0.05 was considered statistically significant. SPSS Statistics 27 (IBM) was used for statistical analysis. Because of the exploratory nature of this study, no correction for α-error accumulation was performed (Supplement 2).
RESULTS
Baseline Characteristics
In total, 297 angiographic evaluations including local 99mTc-MAA injection were performed on 210 patients (91 women and 119 men; mean age ± SD, 59.1 ± 37.3 y) between May 2007 and August 2019 (Fig. 1). Multiple evaluations were performed on 72 patients (2 evaluations in 57 patients, 3 in 7 patients, and 4 in 4 patients). NETs were present in 90.9% (270/297), and neuroendocrine carcinomas in 9.1% (27/297), of all angiographic evaluations. In NET, a tumor grade of 1 was found in 25.6% (76/297), of 2 in 50.5% (150/297), and of 3 in 5.7% (17/297); the grade was unknown in 9.1% (27/297). Hypersecretion symptoms were observed in 32.7% (97/297), and extrahepatic metastases were observed in 41.4% (122/297) (Table 1; Supplemental Table 1). Before angiographic evaluation including 99mTc-MAA injection, different treatments were used in 91.6% (272/297), including external-beam radiotherapy in 1.7% (5/297), primary-tumor surgery in 64.3% (191/297), treatment of hepatic metastases by surgery in 18.2% (54/297), local ablation (e.g., radiofrequency ablation or microwave ablation) in 4.0% (12/297), and TACE or TAE in 8.8% (26/297). PRRT was used in 20.2% (60/297), antibody-based therapy in 2.4% (7/297), somatostatin analog therapy in 57.2% (170/297), prior 90Y RE in 9.8% (29/297), liver transplantation in 0.3% (1/297), chemotherapy in 29.3% (87/297), and targeted therapy in 7.7% (23/297).
Flowchart of analyzed patient cohort.
Baseline Characteristics of All Performed Imaging Evaluations
Imaging Evaluation Including 99mTc-MAA Injection
Before the angiographic evaluation, a CT scan was performed on 84.2% of patients (250/297) and an MRI scan on 24.2% (72/297). A hepatic tumor burden of 25% or less was present in 50.2% (149/297), 25%–50% in 31.0% (92/297), and 50% or more in 16.2% (48/297); the hepatic tumor burden was unknown in 2.7% (6/297). Metastases were hypervascularized in 63.6% (188/297), hypovascularized in 18.2% (54/297), and of a mixed or atypical appearance in 15.5% (46/297). The vascularization type was unknown in 3.0% (9/287).
Before 99mTc-MAA injection, vessel occlusion was necessary in 39.1% (116/297). Preexistent vessel occlusion due to prior coiling was found in 6.1% (18/297) (Table 2; Supplemental Table 2).
Imaging Evaluation Including 99mTc-MAA Injection
A central 99mTc-MAA injection was performed on 17.8% (53/297), a lobar 99mTc-MAA injection on 71% (211/297), and a segmental 99mTc-MAA injection on 2.7% (8/297). Other types of 99mTc-MAA injection were performed on 8.4% (25/297).
Complications occurred in 19 (6.4%) of the 297 patients, with 4 (1.4%) having vascular occlusion, 2 (0.7%) having dissection, and 1 each (0.3%) having the following complications: vasospasm, hypertension, a combination of pain/hypertension and tachycardia, complications associated with contrast medium, and coil dislocation. Complications in 8 additional patients were not specified (2.8%).
A mean lung shunt fraction of 5.7% ± 5.6% was observed in 99mTc-MAA scintigraphy (261 with a lung shunt fraction of ≤10%, 29 with >10%, and 7 with an unknown fraction).
90Y RE
90Y RE was performed after 77.4% of angiographic evaluations including local 99mTc-MAA injection (230/297; glass microspheres, 46.8% [139/297]; resin microspheres, 30.6% [91/297]) across a total of 176 patients (1 treatment, 71.6% [126/176]; 2 treatments, 26.7% [47/176]; 3 treatments, 0.7% [2/176]; 4 treatments, 0.3% [1/176]). For glass microspheres, dosimetry planning was performed using the single-compartment model with a perfused target volume dose of 80–150 Gy. For resin microspheres, the body-surface-area model was used. For resin microspheres, a mean activity of 1.26 ± 0.59 GBq was used for 90Y RE, and for glass microspheres, the mean activity was 3.78 ± 2.24 GBq.
In 44.3% (102/230) of all cases, patients had been treated only by surgery or somatostatin analog therapy before 90Y RE (second-line treatment, Fig. 2). In most other cases (55.7% [128/230]), 90Y RE was performed in a salvage setting after extensive prior therapy.
A 67-y-old man with grade 2 gastric NET and hepatic metastases. Hepatic tumor progression was observed with somatostatin analog treatment. (A) At time of 90Y RE, hepatic tumor burden was less than 25%. (B) In angiographic evaluation, hypervascularized metastases were detected in both liver lobes; thus, same-session sequential therapy of whole liver with 90Y glass microspheres was performed. (C) At follow-up, partial response was observed, with residual metastases in liver segment 4.
90Y RE of the whole liver was used in 44.9% (108/230), and sequential whole-liver therapies was used in separate sessions in 33.1% (76/230). 90Y RE was performed for a single lobe in 19.6% (45/230) and for a selected liver part in 0.4% (1/230).
Second-line 90Y RE after a minimum of prior therapy (including surgery of the primary tumor or hepatic metastasis and somatostatin analog therapy) was used in 44.3% (102/230), whereas extensive prior therapy was used in 55.7% (128/230).
Procedural complications were observed in 4.8% of all cases (11/230, including 3 with vasospasm; 2 each with liver organ necrosis, pain, or stasis of blood flow; and 1 each with sepsis/pain and hemodynamic instability). No complications were observed in 73.7% (219/230). 90Y RE was not performed on 22.6% (67/297) (Table 3; Supplement 3).
Reasons for Canceling 90Y RE
Survival Analysis
Entire Population
At the time of data collection, 59.7% of all patients (105/176) were deceased, 27.3% (48/176) were still alive, 0.4% were lost to follow-up, and in 12.5% (22/176) the last follow-up visit was used for survival analysis. Mean OS after 90Y RE was 38.9 ± 33.0 mo.
After 90Y RE, OS was longer in NET patients (40.8 mo; 95% CI, 27.0–54.7 mo) than in NEC patients (19.3 mo; 95% CI, 8.1–35.1 mo) (Fig. 3A). Differences between the 2 groups were found by the log rank test ( = 6.88, P < 0.009). In NET patients, patients with a grade 1 tumor had a more prolonged median OS (79.3 mo; 95% CI, 42.6–116.0 mo) than patients with a grade 2 tumor (30.6 mo; 95% CI, 25.2–36.0 mo) or grade 3 tumor (21.4 mo; 95% CI, 10.7–32.1 mo) (Fig. 3B). According to the log rank test, the survival distributions for the 3 different tumor grades were statistically significant (
= 6.88, P < 0.009). In NET patients, patients with a grade 1 tumor had a more prolonged median OS (79.3 mo; 95% CI, 42.6–116.0 mo) than patients with a grade 2 tumor (30.6 mo; 95% CI, 25.2–36.0 mo) or grade 3 tumor (21.4 mo; 95% CI, 10.7–32.1 mo) (Fig. 3B). According to the log rank test, the survival distributions for the 3 different tumor grades were statistically significant (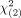 = 10.21, P < 0.006).
= 10.21, P < 0.006).
Kaplan–Meier survival curves investigating influence of 6 different parameters on survival in entire population: tumor type (A), NET tumor grading (B), hepatic tumor burden (C), metastatic vascularization (D), and extrahepatic metastases (E).
An increase in hepatic tumor burden was associated with a reduction in median OS (burden < 25%: 63.2 mo, 95% CI of 36.0–90.4 mo; burden of 25%–50%: 30.3 mo, 95% CI of 25.8–34.8 mo; burden > 50%: 22.5 mo, 95% CI of 16.6–28.4 mo). Significant differences among these 3 survival groups were detected by the log rank test ( = 14.10, P = 0.001) (Fig. 3C).
= 14.10, P = 0.001) (Fig. 3C).
Only slight differences were observed in OS after 90Y RE in patients with different vascularization patterns of hepatic metastases (hypervascularization: 40.5 mo, 95% CI of 17.4–63.6 mo; hypovascularization: 30.3 mo, 95% CI of 20.1–40.5 mo; and mixed appearance: 17.7 mo, 95% CI of 26.5–95.9). The log rank test found no significant differences among the survival distributions for these 3 hepatic vascularization patterns ( = 1.6, P = 0.460) (Fig. 3D).
= 1.6, P = 0.460) (Fig. 3D).
Median OS was similar in patients with extrahepatic metastases (30.6 mo; 95% CI, 19.4–41.8) and without (44.8 mo; 95% CI, 29.8–59.8). No significant differences between the 2 survival groups were observed in the log rank test (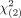 = 0.931, P = 0.335) (Fig. 3E; Supplements 4 and 5; Supplemental Fig. 1).
= 0.931, P = 0.335) (Fig. 3E; Supplements 4 and 5; Supplemental Fig. 1).
Second-Line Therapy Versus Salvage Setting
Patients who received 90Y RE as second-line therapy had a slightly longer OS (44.8 mo; 95% CI, 24.2–65.4 mo) than patients who had 90Y RE in a salvage setting (30.6 mo; 95% CI, 18.5–42.7) (Fig. 4A). However, the log rank test did not yield a statistically significant result ( = 3.109, P = 0.078).
= 3.109, P = 0.078).
Kaplan–Meier survival curves investigating influence of extent of prior therapy (second-line therapy: prior surgery for primary tumor or metastases and somatostatin analog treatment before 90Y RE vs. salvage therapy) on OS (A), hepatic PFS (B), and global PFS (C).
Median hepatic PFS (15.9 mo; 95% CI, 10.6–21.2 mo) and global PFS (14.7 mo; 95% CI, 10.5–18.9 mo) were worse in patients undergoing 90Y RE in a salvage setting than in patients with 90Y RE as second-line therapy (hepatic PFS: 18.6 mo, 95% CI of 14.0–23.2 mo; global PFS: 18.8 mo, 95% CI of 8.3–29.3 mo). The log rank test detected significant differences between the 2 survival groups for hepatic PFS ( = 6.44, P = 0.011) and global PFS (
= 6.44, P = 0.011) and global PFS (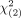 = 6.63, P = 0.010) (Figs. 4B and 4C; Supplement 6; Supplemental Fig. 2).
= 6.63, P = 0.010) (Figs. 4B and 4C; Supplement 6; Supplemental Fig. 2).
In patients receiving 90Y RE as salvage therapy, OS in patients with prior PRRT (30.3 mo; 95% CI, 26.2–34.3 mo) was comparable to that in patients who did not receive prior PRRT (36.2 mo; 95% CI, 19.8–52.5 mo). No significant differences between the 2 groups were found in the log rank test ( = 0.16, P = 0.692) (Fig. 5).
= 0.16, P = 0.692) (Fig. 5).
Kaplan–Meier survival curve investigating influence of prior PRRT on survival in patients receiving 90Y RE as salvage therapy.
DISCUSSION
90Y RE is a possible alternative to a surgical approach to achieve local tumor control in metastatic liver disease. However, alternatives to 90Y RE are manifold, and systemic approaches such as PRRT or new chemotherapies challenge the concept of local liver 90Y RE in general. Therefore, further data are necessary to balance the advantages and disadvantages of all available therapies in NEN patients with hepatic metastases. In this context, the results of this retrospective international multicenter study hold 3 key messages. First, second-line 90Y RE is associated with increased OS and a significant increase in hepatic and global PFS, with OS slightly superior to that seen in published PRRT data. Second, the vascularization type of hepatic metastases does not significantly affect survival after 90Y RE. Third, the presence of extrahepatic metastases in patients with liver-dominant disease does not significantly affect OS in patients undergoing 90Y RE—neither in the entire population nor in patients receiving 90Y RE as second-line therapy.
Successful surgical removal of hepatic metastases is associated with 5-y survival rates of 64%–100% (5,20), but only a minority of patients is eligible for this procedure (5). In patients with increased somatostatin expression who cannot undergo surgery, systemic treatment using somatostatin analogs is considered the treatment of choice in the latest guidelines (9–11). In patients with progressive hepatic metastases, angiographic procedures such as TAE, TACE, or 90Y RE or PRRT are possible treatment options. Although comparative studies among these options are not available, PRRT is recommended as the second-line therapy of choice in the most recent guidelines (9–11). Because of the lack of data, the treatment of sole hepatic metastases or liver-dominant disease remains a controversial topic. In a recent recommendation by Frilling et al., TAE, TACE, or 90Y RE is proposed as a possible alternative in these 2 scenarios (21).
Our study results support these recommendations and further raise the question of whether 90Y RE should be primarily performed in specific scenarios as second-line treatment before PRRT.
We found that patients who had undergone 90Y RE as second-line treatment did show a relevant increase in OS and a significant increase in hepatic and global PFS, compared with patients with a more extensive pretreatment. OS in patients with 90Y RE as second-line treatment was only slightly worse than the results of PRRT in the Rotterdam cohort, with overlapping 95% CIs (44.8 mo, 95% CI of 24.2–65.4 mo, vs. 63 mo, 95% CI of 55–72 mo) (22). Furthermore, the presence of extrahepatic metastases does not influence OS after 90Y RE as second-line therapy. In contrast to 90Y RE in a salvage setting, hepatic tumor burden has no significant impact on OS if 90Y RE is performed as a second-line therapy. Additionally, our data indicate that in 22.6% of all cases, 90Y RE could not be performed because of contraindications associated with advanced hepatic disease such as shunting, a high tumor burden, or liver function deterioration. These findings further suggest that early 90Y RE before PRRT could be beneficial in patients with the liver-dominant disease. In this clinical pathway, a successful local, liver-directed therapy using 90Y RE could be ensured independently of hepatic tumor burden, and the risk of contraindications for 90Y RE caused by advanced oncologic disease such as shunting could be avoided.
In certain scenarios, 90Y RE might yield even further benefits. Although PRRT is limited to patients with increased somatostatin receptor expression compared with the background hepatic uptake (23), this requirement does not hinder 90Y RE. Even hypovascularization of hepatic metastases in contrast-enhanced cross-sectional imaging does not influence OS after 90Y RE. Additional benefits of 90Y RE as second-line therapy in comparison to PRRT are the possibility of continuing somatostatin analog therapy during 90Y RE, the absence of hematologic and renal toxicity, and the possibility of performing 90Y RE as a 1-stop-shop treatment in contrast to the prolonged treatment duration of PRRT (12,22).
Furthermore, the benefits to this therapeutic approach do not seem to be limited to patients without extrahepatic metastases. The presence of extrahepatic metastases in liver-dominant disease did not have a significant impact on OS in our analysis and was not associated with a significant hazard ratio, in contrast to previous studies (12,24).
Hence, our findings stress the need for a prospective study comparing PRRT and 90Y RE as second-line treatment in patients with progressive liver-dominant disease treated with somatostatin analogs.
Our study had some limitations. Because of its retrospective nature and the combined analysis of 90Y RE performed with resin and glass microspheres, differences in the procedural technique could be possible. These limitations could be circumvented only by a prospective study. We did not perform a comparison between 90Y RE and TAE or TACE. Because PRRT is recommended as the therapy of choice, TAE and TACE are considered by some authors as advantageous over 90Y RE to avoid radiation-induced liver failure when PRRT and 90Y RE are combined (25). However, recent findings by Braat et al., as well as our own findings on 90Y RE as salvage therapy in patients with and without prior PRRT, indicate that 90Y RE can be performed safely after PRRT without an increased risk of radiation-induced liver failure (26,27). Furthermore, there are several distinct disadvantages of TAE or TACE compared with 90Y RE, most notably the higher proportion (≤61%) of patients with pain, nausea, or treatment-associated fever (25,28,29) and the need to perform multiple therapies (30). Especially in the current raging coronavirus disease 2019 pandemic, 90Y RE might be advantageous because of the reduced number of procedures necessary to achieve a favorable result and lower therapy-associated complications. However, a randomized trial between TAE or TACE and 90Y RE might improve the acceptance of interventional procedures in general in NET patients with liver-dominant disease.
CONCLUSION
Our results show that 90Y RE has potential as second-line therapy in patients with NENs with liver-dominant disease and thus could be an important alternative to PRRT in certain scenarios. However, a prospective study is necessary to support these promising data.
DISCLOSURE
Benedikt M. Schaarschmidt has received a research grant from PharmaCept. Moritz Wildgruber reports personal fees from Sirtex Medical, outside the submitted work, Roman Kloeckner reports personal fees from Boston Scientific, Bristol-Myers Squibb, Guerbet, SIRTEX, Roche, BTG, Ipsen, Siemens, and MSD–Merck Sharp & Dohme, outside the submitted work, Arthur Braat reports other fees from Terumo and Boston Scientific, outside the submitted work. Manuel Weber is on the speakers bureau for Boston Scientific. Jens Theysohn reports personal fees from BTG, outside the submitted work. No other potential conflict of interest relevant to this article was reported.
KEY POINTS
QUESTION: Is 90Y RE a potential a treatment option in patients with NENs?
PERTINENT FINDINGS: In this retrospective multicenter study, second-line 90Y RE was associated with a significant increase in PFS, with satisfactory OS rates. The presence of extrahepatic metastases in patients with liver-dominant disease undergoing 90Y RE did not affect OS.
IMPLICATIONS FOR PATIENT CARE: This study showed the potential of 90Y RE as a treatment option in patients with NENs with liver-dominant disease and thus could be an important alternative to PRRT.
Footnotes
Published online Sep. 2, 2021.
- © 2022 by the Society of Nuclear Medicine and Molecular Imaging.
REFERENCES
- Received for publication May 11, 2021.
- Revision received August 5, 2021.













