Visual Abstract
Abstract
Limited treatment options in patients with intrahepatic cholangiocarcinoma (iCCA) demand the introduction of new, catheter-based treatment options. Especially, 90Y radioembolization may expand therapeutic abilities beyond surgery or chemotherapy. Therefore, the purpose of this study was to identify factors associated with an improved median overall survival (mOS) in iCCA patients receiving radioembolization in a retrospective study at 5 major tertiary-care centers. Methods: In total, 138 radioembolizations in 128 patients with iCCA (female, 47.7%; male, 52.3%; mean age ± SD, 61.1 ± 13.4 y) were analyzed. Clinical data, imaging characteristics, and radioembolization reports, as well as data from RECIST, version 1.1, analysis performed 3, 6, and 12 mo after radioembolization, were collected. mOS was compared among different subgroups using Kaplan–Meier curves and the log-rank test. Results: Radioembolization was performed as first-line treatment in 25.4%, as second-line treatment in 38.4%, and as salvage treatment in 36.2%. In patients receiving first-line, second-line, and salvage radioembolization, the disease control rate was 68.6%, 52.8%, and 54.0% after 3 mo; 31.4%, 15.1%, and 12.0% after 6 mo; and 17.1%, 5.7%, and 6.0% after 1 y, respectively. In patients receiving radioembolization as first-line, second-line, and salvage treatment, mOS was 12.0 mo (95% CI, 7.6–23.4 mo), 11.8 mo (95% CI, 9.1–16.6 mo), and 8.4 mo (95% CI, 6.3–12.7 mo), respectively. No significant differences among the 3 groups were observed (P = 0.15). Hepatic tumor burden did not significantly influence mOS (P = 0.12). Conclusion: Especially in advanced iCCA, second-line and salvage radioembolization may be important treatment options. In addition to ongoing studies investigating the role of radioembolization as first-line treatment, the role of radioembolization in the later treatment stages of the disease demands further attention.
Cholangiocarcinoma is a rare, aggressive malignancy that accounts for approximately 3% of all gastrointestinal tumors and is associated with low median overall survival (mOS) rates (1,2). Surgery remains the most promising approach, although recurrence rates are high (2–6). In patients who have recurrent or metastatic disease or are ineligible for resection, therapeutic options are limited. In such cases, cytotoxic chemotherapy with modest response rates is the standard of care (4,6). Because other treatment options such as radiotherapy or thermal ablation are confined to small, localized tumors, various catheter-based treatment options have been proposed to overcome this troubling situation, most notably for intrahepatic cholangiocarcinoma (iCCA) (4,5,7). For this use, hepatic artery infusion, transarterial chemoembolization (TACE), and radioembolization have gained particular considerate attention.
In hepatic artery infusion, a high hepatic dose of a chemotherapeutic agent is achieved by local administration of a chemotherapeutic agent in the hepatic artery. Vascular access can be achieved either by surgical placement of a hepatic arterial infusion pump or by interventional catheter placement via the femoral artery. mOS of up to 25.0 mo has been reported (8,9).
TACE, on the other hand, relies on the combined effect of vessel occlusion at a capillary level and local administration of a chemotherapeutic agent. Promising results have been reported in recent metaanalyses, indicating an mOS of up to 15.9 mo in a selected patient cohort (10–12). In addition to conventional TACE, other embolic agents such as degradable starch microspheres or drug-eluting beads have been proposed. Because of small patient cohorts, however, a generalized recommendation on these new embolic agents cannot be derived from the available data (13–15).
Radioembolization uses the increased vasculature of intrahepatic tumors in comparison to hepatic tissue (16,17). Radioactive microspheres that have been previously injected into the hepatic artery accumulate predominantly in the capillary bed of tumors. The short range of β−-radiation emitted by 90Y ensures highly effective control of the local tumor, whereas the surrounding tissue is spared. Before radioembolization, pretherapeutic angiography followed by local injection of 99mTc-labeled macroaggregated albumin is mandatory to exclude extrahepatic shunting. With an mOS of up to 14.3 mo, the results are comparable to those for TACE (10–12,18,19).
Because of the lack of prospective, comparative studies, the overall evidence for each of these treatment regimens is low. Current recommendations are derived mostly from metaanalyses that are based on a variety of prospective and retrospective datasets with low patient numbers (10,11). In the advent of prospective studies focusing on early radioembolization, it is crucial to identify other potential applications for radioembolization in larger cohorts (20). Therefore, the aim of this multicenter retrospective study was to analyze the impact of clinical and tumor-associated factors on mOS. Such research will facilitate patient selection for radioembolization in the future and further the applications for this promising technique in iCCA patients beyond early radioembolization.
MATERIALS AND METHODS
Retrospectively, we collected data on patients with cholangiocarcinoma who underwent radioembolization at any of 5 major tertiary-care centers in Germany during the 14 y from May 2007 to May 2021. To be included, the patients had to have histopathologically proven iCCA and had to have undergone radioembolization. Exclusion criteria were extrahepatic cholangiocarcinomas such as Klatskin tumors or gallbladder carcinomas.
At each center, a standardized questionnaire was used to obtain all necessary data (supplemental data, section 1; supplemental materials are available at http://jnm.snmjournals.org). Tumor response was assessed according to RECIST, version 1.1, at the corresponding centers to obtain the disease control rate (DCR) after radioembolization (21). Then, data from each center were anonymized and merged into a single database for further statistical analysis. The institutional ethic committee of the University Duisburg–Essen approved the study (application 20-9747-BO) on June 7, 2021. Because the analysis was retrospective, the need for informed consent was waived.
Statistical Analysis
OS was the primary endpoint of this study. Kaplan–Meier curves, including log-rank tests, were used to investigate the impact of tumor type, hepatic tumor burden, and tumor vascularization pattern; the presence of extrahepatic metastases and tumor-accompanying ailments such as ascites and cirrhosis; the administered microsphere type; and the line of therapy (first-line, second-line, or salvage therapy). Additionally, a multivariable Cox regression analysis was performed (supplemental data, section 2).
A P value of less than 0.05 indicated statistical significance. Because the study was explorative, no α-error correction was performed. SPSS Statistics (version 27; IBM) and R (version 4.2.0; R Core Team, 2022) were used for statistical analysis.
RESULTS
Baseline Characteristics
Data on 184 radioembolizations in 142 patients were collected from all 5 centers. Seventeen cases did not match the inclusion criteria and were excluded from further analysis (Fig. 1). In 29 cases, the liver was treated in 2 separate sessions, which were considered as 1 treatment. Therefore, 138 radioembolizations in 128 patients were eligible for further analysis (female, 47.7% [61/128]; male, 52.3% [67/128]; mean height [±SD], 172.1 ± 9.5 cm; mean weight, 75.3 ± 17.1 kg; mean age, 61.1 ± 13.4 y). In 10 cases, radioembolization was repeated because of relapse (Table 1; supplemental data, section 3).
Flowchart of analyzed patients. CCA = cholangiocarcinoma; HCC = hepatocellular carcinoma.
Baseline Characteristics of All Radioembolizations
Before radioembolization, other treatments had been conducted on 84.6% (103/138). In total, 25.4% (35/138) received first-line radioembolization without any prior therapy, 38.4% (53/138) received second-line radioembolization, and 36.2% (50/138) received salvage radioembolization after multiple prior therapies (Table 2). Section 4 of the supplemental data provides further information on baseline characteristics, preinterventional imaging, and radioembolization.
Treatments Before Radioembolization
Follow-up Examinations
For the entire cohort, follow-up examinations were performed after 3 mo (mean, 86 ± 49 d), 6 mo (mean, 166 ± 78 d), and 1 y (mean, 346 ± 81 d). DCR for the entire cohort was 57.2% (79/138) after 3 mo, 18.1% (25/138) after 6 mo, and 6.5% (9/138) after 1 y. In patients receiving first-line radioembolization, DCR was 68.6% (24/35) after 3 mo, 31.4% (11/35) after 6 mo, and 17.1% (6/35) after 1 y. In patients receiving second-line radioembolization, DCR was 52.8% (28/53) after 3 mo, 15.1% (8/53) after 6 mo, and 5.7% (3/53) after 1 y. In patients receiving radioembolization as a salvage treatment, however, DCR was 54.0% (27/50) after 3 mo, 12.0% (6/53) after 6 mo, and 6.0% (3/50) after 1 y.
Survival Analysis
mOS in the entire cohort was 10.7 mo (95% CI, 8.4–12.7 mo). One-year OS was 45.3% (95% CI, 37.2–55.2 mo), and 2-y OS was 16.7% (95% CI, 10.8–25.9 mo). At the time of the analysis, 75.4% (104/138) were deceased, 10.9% (15/138) were alive, and 13.7% (19/138) were lost to follow-up.
To identify potential differences among different subgroups, a further analysis was performed with a focus on the following tumor characteristics: tumor type (diffuse vs. mass-forming), hepatic tumor burden, and tumor vascularization pattern. Mass-forming tumors had a significantly longer survival than tumors with a diffuse growth pattern (mass-forming, 12.0 mo [95% CI, 9.5–15.9 mo]; diffuse growth pattern, 7.6 mo [95% CI, 5.8–12.7 mo], 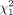 [n = 136] = 4.3, P = 0.038, Fig. 2A). A lower hepatic tumor burden tended to be associated with an increased mOS (<25%, 11.8 mo [95% CI, 9.1–15.6 mo]; 25%–50%, 8.0 mo [95% CI, 5.8–16.6 mo]; >50%, 6.4 mo [95% CI, 4.2–16.3 mo]). However, no statistical differences among the subgroups were detected by the log-rank test (
[n = 136] = 4.3, P = 0.038, Fig. 2A). A lower hepatic tumor burden tended to be associated with an increased mOS (<25%, 11.8 mo [95% CI, 9.1–15.6 mo]; 25%–50%, 8.0 mo [95% CI, 5.8–16.6 mo]; >50%, 6.4 mo [95% CI, 4.2–16.3 mo]). However, no statistical differences among the subgroups were detected by the log-rank test ( [n = 128] = 4.2, P = 0.12, Fig. 2B). mOS was 12.7 mo (95% CI, 9.8–17.0 mo) for hypervascularized tumors, 8.0 mo (95% CI, 5.4–12.0 mo) for hypovascularized tumors, and 9.1 mo (95% CI, 8.1–16.9) for tumors with a mixed appearance. According to the log-rank test, a slight significant difference in OS existed among the 3 subgroups (
[n = 128] = 4.2, P = 0.12, Fig. 2B). mOS was 12.7 mo (95% CI, 9.8–17.0 mo) for hypervascularized tumors, 8.0 mo (95% CI, 5.4–12.0 mo) for hypovascularized tumors, and 9.1 mo (95% CI, 8.1–16.9) for tumors with a mixed appearance. According to the log-rank test, a slight significant difference in OS existed among the 3 subgroups ( [n = 129] = 6.6, P = 0.037; Fig. 3).
[n = 129] = 6.6, P = 0.037; Fig. 3).
A 64-y-old man with stage 4 iCCA with lymph node metastases. (A and B) Initial MRI shows hypovascularized tumor in right liver lobe (A, arrow), with faint contrast agent uptake in preinterventional angiography with subsequent 99mTc-macroaggregated albumin injection (B, arrow). Radioembolization of right hepatic artery with 4.64 GBq was performed 27 d after 99mTc-macroaggregated albumin injection. (C) Postinterventional MRI 126 d after radioembolization showed partial tumor response (arrow). Patient died after 176 d.
Kaplan–Meier survival curves for different tumor characteristics in analyzed cohort: tumor type (A), hepatic tumor burden (B), and tumor vascularization pattern (C).
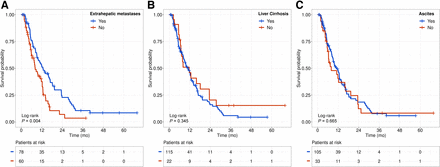
Patients with extrahepatic metastases had a significantly shorter mOS than patients without distant metastases (extrahepatic metastases, 8.1 mo [95% CI, 6.4–12.2 mo]; no extrahepatic metastases, 12.7 mo [95% CI, 9.2–18.2 mo], 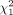 [n = 138] = 8.3, P = 0.004). However, mOS was significantly influenced neither by the presence of liver cirrhosis (present, 11.7 mo [95% CI, 6.8–23.5 mo]; absent, 11.0 mo [95% CI, 8.4–13.7 mo];
[n = 138] = 8.3, P = 0.004). However, mOS was significantly influenced neither by the presence of liver cirrhosis (present, 11.7 mo [95% CI, 6.8–23.5 mo]; absent, 11.0 mo [95% CI, 8.4–13.7 mo];  [n = 137] = 0.9, P = 0.34) nor by the presence of ascites (present, 8.0 mo [95% CI, 6.3–19.4 mo]; absent, 11.0 mo [95% CI, 9.0–13.7 mo];
[n = 137] = 0.9, P = 0.34) nor by the presence of ascites (present, 8.0 mo [95% CI, 6.3–19.4 mo]; absent, 11.0 mo [95% CI, 9.0–13.7 mo];  [n = 138] = 0.2, P = 0.66) (Fig. 4).
[n = 138] = 0.2, P = 0.66) (Fig. 4).
Kaplan–Meier survival curves for different baseline characteristics in analyzed cohort: extrahepatic metastases (A), liver cirrhosis (B), and ascites (C).
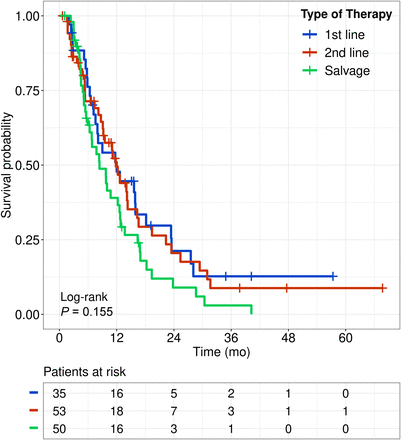
In the analyzed cohort, radioembolization was characterized as first-line (no prior therapy), second-line (only one kind of prior therapy), or salvage treatment (at least 2 prior therapies). In patients receiving radioembolization as first- and second-line treatment, mOS was 12.0 mo (95% CI, 7.6–23.4 mo) and 11.8 mo (95% CI, 9.1–16.6 mo), respectively. In patients receiving radioembolization as salvage therapy, mOS was 8.4 mo (95% CI, 6.3–12.7 mo). No significant differences among the 3 groups were detected by the log-rank test ( [n = 138] = 3.7, P = 0.15; Fig. 5; supplemental data, section 4).
[n = 138] = 3.7, P = 0.15; Fig. 5; supplemental data, section 4).
Kaplan–Meier survival curves for different treatment lines in analyzed cohort.
Cox Regression
The assumption of proportional hazards of the Cox model was met globally (P = 0.55) and by all variables (P > 0.05). The analysis revealed that the presence of extrahepatic metastases (hazard ratio [HR], 1.99 [95% CI, 1.25–3.2]; P = 0.004) and vascularization patterns other than hypervascularization (hypovascularization: HR, 2.27 [95% CI, 1.35–3.8]; P = 0.002) (mixed: HR 2.21 [95% CI, 1.15–4.2]; P = 0.017) were statistically significant predictors of a worse mOS (Fig. 6). Although higher HRs were observed for a higher hepatic tumor burden, no significant differences could be observed (25%–50%: HR, 1.46 [95% CI, 0.82–2.6]; P = 0.197) (>50%: HR, 1.60 [95% CI, 0.77–3.3]; P = 0.209).
Forest plot for multivariable Cox regression analysis. dof = degrees of freedom.
DISCUSSION
To aid in making clinical decisions on iCCA patients, it is key to have a large retrospective study that identifies factors favoring radioembolization. Four main factors were identified in our multicenter study, comprising, to our knowledge, the largest cohort of iCCA patients treated with radioembolization to date. First, second-line or salvage radioembolization of iCCA patients is a viable option, with promising DCRs, and might have a favorable effect on mOS. Second, mOS is significantly longer in iCCA patients with hypervascularized tumors than in iCCA patients with other tumor types. Third, even a high tumor burden does not lead to significant changes in mOS. Fourth, extrahepatic metastases have a significant impact on mOS in iCCA patients.
Despite relevant advances in oncology, treatment of iCCA remains challenging. At the moment, surgical treatment has to be considered the most favorable option. If complete tumor removal can be achieved, an mOS of up to 45.1 mo can be achieved in iCCA patients in general (6). In patients with resected iCCA, 5-y survival rates of up to 63% have been published (22,23). Despite the introduction of adjuvant radio- or chemotherapy, R0 resection remains a prerequisite for such a favorable outcome. However, because of the necessary extent of tumor removal, R0 resection can be achieved in approximately only 36% of cases. Chemotherapeutic regimens for iCCA are scarce (4,5). Hence, the lack of further therapeutic options leads to a relevant decrease in mOS if chemotherapy fails or patients are ineligible for medical therapy: although active palliative treatment still has an mOS of 10.6 mo, an mOS of only 4.0 mo for best supportive care was reported in the recent analysis of the European Network for the Study of Cholangiocarcinoma (ENSCCA) registry by Izquierdo-Sanchez et al. (6).
Therefore, expanding therapeutic options is of the utmost importance. Especially in liver-dominant disease, minimally invasive, catheter-based therapies such as hepatic artery infusion, TACE, or radioembolization might be important adjuncts to the contemporary arsenal (8–12). Although a comparative study between TACE and radioembolization has been started by Kloeckner et al., the results have not been published yet (24). As a result, no comparative studies between these options are available to date and no relevant differences between them have been reported in the available metaanalyses (11,12). However, distinct advantages of radioembolization are a favorable safety profile and excellent patient comfort (25). In comparison to TACE, postinterventional infections after radioembolization are exceptionally rare, and the short-term toxicity caused by postembolization syndrome can be drastically reduced (26,27). Our data confirm the potential of radioembolization as second-line treatment and in a salvage setting. Even in heavily pretreated patients, mOS after radioembolization was 8.4 mo, which is considerably higher than the mOS of patients receiving best supportive care in the ENSCCA registry (6). Furthermore, mOS in patients receiving salvage radioembolization did not differ significantly from that in patients receiving second-line radioembolization in our cohort and showed no relevant aberrations from previously published data on second-line radioembolization (25,28).
In this cohort, a significantly higher mOS was seen in patients with mass-forming and hypervascularized tumors. This observation is most likely associated with an increased accumulation of microspheres in the vasculature of the tumor—an accumulation that is far more distinct than in hypovascular masses or tumors with a diffuse growth pattern. These findings are interesting additions to those of Willowson et al., who found that a high total lesion glycolysis at baseline and a low tumor heterogeneity in 18F-FDG uptake were considered as predictors of treatment response (29). In recent studies, Bourien et al. and Manceau et al. found that tumor doses higher than 158 and 260 Gy, respectively, were predictive of tumor response in patients treated with and without concomitant chemotherapy and radioembolization (30,31). Henceforth, these findings may provide the basis for further inquiries into the role of personalized dosimetry in patients with iCCA. However, we did not find high hepatic tumor burden to have a negative impact on mOS as previously described by Paprottka et al. (25), thus confirming the preliminary results of Köhler et al. (20). Additionally, as previously reported, neither the presence of ascites nor liver cirrhosis seems to influence mOS after radioembolization (20,25).
In contrast to the study by Köhler et al., the presence of extrahepatic metastases had a significant impact on mOS in our cohort. Thus, our results are in accordance with a study by Jia et al., who observed, in a univariable Cox regression model, that the presence of lymph node metastases was significantly associated with survival (28). The most probable explanation for this finding is the notably larger patient cohort in the present study (20). Although this finding might be interpreted as an exclusion criterion for radioembolization, even in patients with liver-dominant disease, the lack of other treatment options could still lead to considerate gains in OS in comparison to best supportive care in this scenario.
At first sight, the results of first-line radioembolization have to be considered sobering. The observed mOS of 12.0 mo after first-line radioembolization is considerable lower than found in previously published surgical studies or the ENSCCA registry (6,22,23). Additionally, other retrospective studies on first-line radioembolization, such as a recent study by Buettner et al., reported an mOS of 16 mo (26). However, it is highly probable that radioembolization was reserved for patients ineligible for surgery or chemotherapy in our cohort, resulting in a potential selection bias. Still, as a minimally invasive procedure with limited side effects, first-line radioembolization may be a valuable option in patients who can neither undergo surgery nor receive chemotherapy. Because the literature on early radioembolization is limited (32), the final verdict on early radioembolization will depend on the overdue results of prospective studies such as the SIRRCA trial (NCT02807181).
The limitations of the study are caused mainly by its retrospective nature. In particular, retrospective data on iCCA patients treated with first-line radioembolization in the current clinical setting have to be interpreted with extreme caution. As first-line radioembolization is not incorporated in the current guidelines, it is possible that mainly patients with comorbidities underwent this procedure in our cohort, which might result in a considerable selection bias. Further comparative studies are necessary before the value of first-line radioembolization can be judged appropriately. Additionally, differences in patient selection cannot be circumvented in the setting of the current study, stressing the need for further prospective investigations.
Low enrollment rates per year might have led to worse outcomes due to lack of user experience. However, all centers performed radioembolizations regularly for other tumors, such as hepatocellular carcinoma, leading to a high level of expertise at each participating center.
CT was the main imaging modality used in our cohort, albeit MRI is considered far more sensitive in evaluating local tumor spread in iCCA (4). Hence, an underestimation of tumor extent before radioembolization may be possible in the present cohort and may impact mOS analysis. As this analysis focused solely on radioembolization, and other treatment modalities were not investigated, a distinct recommendation between hepatic artery infusion, TACE, and radioembolization cannot be derived from the present dataset. However, the findings of this analysis may provide an important basis for the design of forthcoming studies. Furthermore, the retrospective nature of this study thwarted the analysis of personalized dosimetry. On the basis of the SPECT/CT scan after 99mTc-macroaggregated albumin injection, the injected 90Y activity can be adapted to ensure a preferably high radiation dose without damaging adjacent, nontumorous liver tissue. This concept may be of considerable value in tumors such as iCCA that are notoriously difficult to treat.
CONCLUSION
Second-line and salvage radioembolization may be an important option in advanced iCCA, independent of the hepatic tumor burden or the presence of tumor-accompanying ailments such as ascites or liver cirrhosis. In the onset of studies investigating the role of first-line radioembolization, the potential benefits of radioembolization in the later treatment stages of the disease should not be underestimated. Here, further investigation in prospective studies is necessary.
DISCLOSURE
Benedikt Schaarschmidt and Jens Theysohn received a research grant from PharmaCept for an ongoing investigator-initiated study not related to this paper. Roman Kloeckner is a consultant for Boston Scientific, Bristol-Myers Squibb, Guerbet, and SIRTEX and received lecture fees from AstraZeneca, BTG, Guerbet, Ipsen, SIRTEX, and MSD–Merck Sharp & Dohme. Daniel Pinto dos Santos is a consultant for Cook Medical and received lecture fees from Bayer. Manuel Weber is a consultant for Boston Scientific, Terumo, Advanced Accelerator Applications, and Eli Lilly. Jens Theysohn is a consultant for Boston Scientific, PharmaCept, Guerbet, and Roche. No other potential conflict of interest relevant to this article was reported.
KEY POINTS
QUESTION: What are beneficial scenarios for radioembolization in cholangiocarcinoma patients?
PERTINENT FINDINGS: In this multicenter retrospective study, we showed that second-line or salvage radioembolization were viable options, with promising DCRs and a possibly favorable effect on mOS. In particular, hypervascularized tumors showed a statistically significant longer mOS than other tumor types.
IMPLICATIONS FOR PATIENT CARE: Second-line and salvage radioembolization may be important treatment options in advanced cholangiocarcinoma, independent of hepatic tumor burden, ascites, or liver cirrhosis. In addition to studies investigating the role of first-line radioembolization, the potential benefits of radioembolization in later treatment stages should be investigated in prospective studies.
ACKNOWLEDGMENTS
This publication contains parts of the doctoral thesis of Thomas Dertnig and is therefore in partial fulfilment of the requirements for an MD thesis at the Medical Faculty of the University Duisburg–Essen. Helin Durnus assisted with data collection at the Department of Diagnostic and Interventional Radiology, University of Cologne.
Footnotes
Published online Nov. 3, 2022.
- © 2023 by the Society of Nuclear Medicine and Molecular Imaging.
REFERENCES
- Received for publication June 30, 2022.
- Revision received October 27, 2022.