Abstract
1609
Objectives: Monoamine oxidases play a significant role in the pathogenesis of neurodegeneration. The role of the brain isozyme, MAO-A in neurodegeneration is less known. We report evaluation of our newly developed fluoroalkylated azaindole, [18F]FAZIN3 as a novel, reversibly binding PET imaging agent for MAO-A in Alzheimer’s disease (AD) and Parkinson’s disease (PD). [18F]FAZIN3 was compared to the known MAO-A PET radiotracer, [18F]Fluoroethyl Harmol ([18F]FEH) to further support the role of MAO-A in AD and PD. Human post-mortem brain autoradiographic studies of [18F]FAZIN3 and [18F]FEH were carried out in cognitively normal (CN) controls, AD, and PD brains containing anterior cingulate (AC) and corpus callosum (CC).
Methods: [18F]FAZIN3 and [18F]FEH were prepared by reacting the tosylate precursors using high specific activity [18F]fluoride from PETNET. [18F]FAZIN3 and [18F]FEH were purified by HPLC and used for in vitro studies. Human post-mortem brain tissues consisted of AC (grey matter, GM) and CC (white matter, WM). Brain slices (10 μm thick) were obtained on a Leica 1850 cryotome. Controls (CN), n=6; age 81-90 total Tangle=1 to 3.5, no Lewy body (LB); PD, n=6, age 77-89, total Tangle= 2 to 7, LB III-IV; AD, n=6, age 77-89, total Tangle = 10 to 15; no LB. Brain slices were incubated [18F]FAZIN3 or [18F]FEH (approx. 1 μCi/cc) in PBS (pH 7.4) buffer at 25oC for 60 min and subsequently washed with PBS buffer. Nonspecific binding was measured using 10 μM clorgyline. Tau binding by [18F]FAZIN3 was confirmed in the presence of MK-6240 (10 μM). Optiquant program, was used for regions of interest analysis and digital light units/ mm2 (DLU/mm2) used to quantify percentage binding of [18F]FAZIN3 or [18F]FEH. Immunostaining with anti-Tau, anti-Aβ and ubiquitin for LB was done on adjacent sections of all subjects. [125I]IPPI, a Tau-binding radiotracer and [18F]Flotaza, an Aβ plaque binding PET radiotracer, developed in our laboratory were used in adjacent slices to confirm immunostaining results and correlate with [18F]FAZIN3 and [18F]FEH binding in the AD brains.
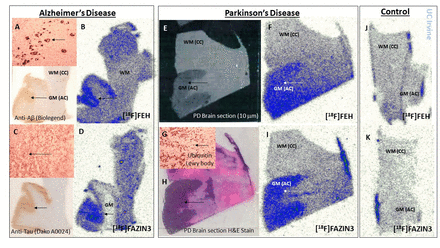
Results: The gray matter (GM) were clearly delineated in the AD autoradiographic images of [18F]FEH (Fig-1B) and [18F]FAZIN3 (Fig-1D), with an average GM/WM>2 for [18F]FEH and GM/WM>3 for [18F]FAZIN3. The binding correlated well with the high binding of [125I]IPPI to Tau (GM/WM>4) and [18F]Flotaza to Aβ plaques (GM/WM>100) consistent with cortical binding corresponding to anti-Aβ (Fig-1A) and anti-Tau (Fig-1C). All CN subjects exhibited lower [18F]FEH and [18F]FAZIN3 binding in the GM (Fig-1J,K) compared to the AD and PD subjects. Ubiquitin confirmed presence of LB (Fig-1G). All PD subjects exhibited significantly higher binding of [18F]FEH (Fig-1F) and [18F]FAZIN3 (Fig-1I) in GM compared to CN subjects. Average GM/WM=1.5-2 for [18F]FEH and GM/WM>3 for [18F]FAZIN3 for the PD subjects. Nonspecific binding of [18F]FEH was found to be higher than [18F]FAZIN3. Conclusion: Increased [18F]FEH and [18F]FAZIN3 binding in PD and AD brains compared to CN brain samples suggests a significant upregulation of MAO-A in neurodegeneration.Figure-1: Binding of [18F]FEH and [18F]FAZIN3 in post-mortem AD, PD and CN human brain tissue. Figs. 1A-D are AD brain sections of the same AD subject showing anti-Aβ immunostain (A), [18F]FEH binding autoradiographs in the grey matter (GM) regions (B), anti-Tau immunostain of adjacent sections (C), and [18F]FAZIN3 binding autoradiographs in the GM regions (D). Figs. 1E-I are PD brain sections of the same PD subject showing PD brain section with white matter (WM, corpus callosum, CC) (E), [18F]FEH binding autoradiographs in the grey matter (GM) regions (F), ubiquitin staining of Lewy bodies (G), H&E staining of adjacent section (H) and [18F]FAZIN3 binding autoradiographs in the GM regions (I). Fig. 1J is [18F]FEH binding autoradiographs of control subject and Fig.1K is [18F]FAZIN3 binding autoradiograph of the same control subject.