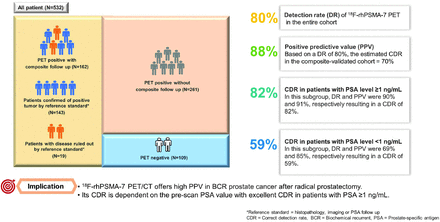
Visual Abstract
Abstract
The objective of this retrospective study was to assess the detection rate (DR), positive predictive value (PPV), and correct detection rate (CDR) of 18F-rhPSMA-7 PET/CT in biochemical recurrence (BCR) of prostate cancer (PCa) after radical prostatectomy (RP) using composite validation. Methods: 18F-rhPSMA-7 PET/CT scans of patients with BCR between July 2017 and June 2018 were retrospectively reviewed. All suspicious lesions were recorded. The reference standard was histopathology or combinations of histopathology, imaging, or prostate-specific antigen (PSA) follow up, defined as composite reference standard. DR was calculated as the proportion of PSMA PET–positive patients to all patients independent of the reference standard, whereas the CDR was the percentage of patients who had at least 1 true-positive PSMA PET lesion localized that corresponded with the reference standard. The PPV was defined as the proportion of patients who had true-positive to all positive findings. The correlation between DR and patient characteristics was evaluated. Results: A total of 532 patients with a median PSA level of 0.97 ng/mL (interquartile range: 0.41–2.46 ng/mL) were included. Of these, 162 patients had composite follow-up at a median duration of 5.6 mo (range: 1.1–14.2 mo). The proportion of patients who had no lesion visualized on PET/CT, localized disease, and any distant metastases (M1) were 20%, 43%, and 37%, respectively. PET DR among all patients was 80%. On a per-patient basis, the PPV of 18F-rhPSMA-7 PET/CT in the composite cohort was 88%, and the CDR was 70%. The PPV in the histopathology-proven cohort was 91%, and the CDR in this subgroup was 73%. In patients with PSA levels ≥ 1 ng/mL the DR and PPV were 90% and 91%, respectively, resulting in a CDR of 82%. In patients with PSA levels < 1 ng/mL, the DR and PPV were 69% and 85%, respectively, resulting in a CDR of 59%. There was a significant positive correlation between 18F-rhPSMA-7 PET/CT detection efficacy and stratified PSA levels (P = 0.005), as well as PSA nadir after prostatectomy (P < 0.001). Conclusion: 18F-rhPSMA-7 PET/CT offers high PPV in BCR after RP. Its CDR is dependent on the prescan PSA value with excellent CDR in patients with PSA ≥ 1 ng/mL.
- biochemical recurrence
- PET
- prostate-specific membrane antigen
- correct detection rate
- positive predictive value
Biochemical recurrence (BCR) of prostate cancer (PCa) after initial curative treatment is frequent, especially in patients with high-risk disease. Early and localized detection of recurrent PCa is essential to plan further local or systemic treatment. Early initiation of localized salvage therapy in localized disease lowers the risk of metastasis and decreases PCa-specific mortality (1,2). Failure of salvage treatments is known to be related to incomplete tumor localization and therefore inadequate treatment strategies (1).
Radiolabeled-prostate-specific membrane antigen (PSMA) PET/CT imaging is currently regarded as the most sensitive and precise imaging modality for localization of BCR of PCa (3,4). Multiple prospective and retrospective studies and recent meta-analyses indicate a high accuracy of 68Ga-PSMA-11 (5–8). Currently, 18F-labeled PSMA-ligands are increasingly being evaluated given their advantages such as longer half-life and with this facilitated large-scale production for broad distribution. Further they offer a potentially higher image resolution due to lower positron range when compared with 68Ga-labeled agents. They showed excellent diagnostic performance in men with BCR of PCa (9–11), but the clinical impact is yet to be explored (12). 18F-rhPSMA-7 is one of a new class of radiohybrid (rh) PSMA-ligands that provides the unique opportunity of both fluorination and chelation using radiometals (68Ga, 177Lu-177).
Data on detection rate (DR) of 18F-rhPSMA-7 in BCR after radical prostatectomy (RP) have recently been published (11). However, the report is lacking information on the validation of positive lesions, which is important to assess the diagnostic accuracy.
Our aim was to establish the positive predictive value (PPV) and the correct detection rate (CDR) validated by a standard of truth for 18F-rhPSMA-7 PET/CT in an expanded cohort of patients with BCR after radical prostatectomy.
MATERIALS AND METHODS
Patients
A total number of 532 patients who underwent a clinically indicated 18F-rhPSMA-7 PET/CT for BCR between July 2017 and June 2018 were retrospectively evaluated. A subcohort of the patients in this work was already included in a prior analysis (11). Patients who have undergone RP either as a primary treatment or a salvage treatment after external-beam radiation therapy with curative intent were included. Clinical data are presented in Table 1. The median PSA level at the time of 18F-rhPSMA-7 PET/CT was 0.97 ng/mL (interquartile range [IQR]: 0.41–2.46 ng/mL).
Patient Characteristics
All patients gave written informed consent before the 18F-rhPSMA-7 PET/CT imaging. All reported investigations were conducted in accordance with the Helsinki Declaration and with national regulations. The retrospective analysis was approved by the local ethics committee (permit 290/18S), and the requirement to obtain informed consent for data collection was waived. The administration of 18F-rhPSMA-7 complied with The German Medicinal Products Act, AMG §13 2b, and the responsible regulatory body (Government of Oberbayern).
18F-rhPSMA-7 PET/CT Imaging and Image Interpretation
18F-rhPSMA-7 was synthesized and PET/CT scans were obtained as described previously (11). In brief, PET scans were acquired in 3-dimensional mode, together with intravenous and oral contrast-enhanced CT. The mean activity of 18F-rhPSMA-7 was 331 MBq (IQR: 296–364 MBq), and the mean uptake time was 80 min (IQR: 67–89 min). Images were reviewed by an experienced, board-certified nuclear medicine physician and a board‐certified radiologist. The Prostate Cancer Molecular Imaging Standardized Evaluation (PROMISE) criteria were used for lesion assessment (13). All suspicious lesions were categorized into 4 regions (prostate bed, pelvic nodes, extrapelvic nodes/nonbone metastases, and bone metastasis), with a total of 21 subregions, using the miTNM framework (13).
Lesion Detection and Validation
DR was defined as the proportion of PSMA PET–positive patients to all patients independent of the reference standard, whereas the CDR was the percentage of patients who had at least 1 true PSMA PET–positive lesion localized that corresponded with a reference standard. The PPV was the proportion of patients who had true-positive findings divided by any positive.
The follow-up data, including proven histopathology (either by biopsy or surgery), imaging, or PSA follow-up after local/focal therapy, of all patients were reviewed. The combinations of above-mentioned follow-up data (priority in descending order) were defined as composite reference standard. The validated positive lesions in 18F-rhPSMA-7 PET were recorded as true- or false-positive. True-negative was not defined.
Statistical Analysis
DRs were compared in each stratified PSA value subgroup using the Pearson χ2 test with a 2-sided significance level of 0.05. True- and false-negative regions could not be determined because PET-negative regions were not be biopsied or followed up on, thus the specificity of the test could not be calculated. Statistical analysis was performed with MedCalc software (version 13.2.0, 2014; MedCalc).
RESULTS
PPV and CDR
The DR of 18F-rhPSMA-7 PET in the entire cohort was 80% (423/532 patients, Fig. 1). From all patients, 162 of 532 patients (30%) had follow-up after PET with a median duration of 5.6 mo (range: 1.1–14.2 mo). Most of these patients had follow-up based on imaging (115 patients, 71%), whereas 22 patients (14%) and 25 patients (15%) had histopathologic and PSA follow-up after targeted treatment, respectively. PSA follow-up was used as a part of the lesion validation in patients who received external-beam radiation as focal salvage therapy without systemic therapy. The detailed patients’ characteristics and PSA response in this subgroup are shown in Supplemental Table 1 and Supplemental Figure 1 (supplemental materials are available at http://jnm.snmjournals.org), respectively. A patient example is shown in Figure 2.
Flow diagram for efficacy cohort with composite validation.
True-positive pelvic lymph nodes metastasis. Axial fused PET/CT (A) and CT (B) revealed 2 subcentimeter lymph nodes with 18F-rhPSMA-7 uptake (SUVmax = 8.7) at left presacral region. Largest one measured about 6.3 mm in short-axis diameter (arrow). After external-beam radiation, follow-up axial fused PET/CT (C) and CT (D) showed significantly decreased size of aforementioned lymph node, measuring about 3 mm (SUVmax = 3.3). This case was recorded as true-positive according to size decrease > 30% after targeted therapy with minimum size change of 3 mm.
In the composite-validated cohort, 143 of 162 patients (88%) had been validated as true-positive, resulting in a per-patient PPV of 88% (95% CI: 82%–92%). An example is shown in Supplemental Figure 2. On the basis of a DR of 80%, the estimated CDR in the composite-validated cohort was 70%. For the histopathologic-validated subgroup, the per-patient PPV was 91% (95% CI: 72%–97%), and the estimated CDR was 73%. PPVs according to a region-based analysis in both validated subgroups are demonstrated in Table 2.
PPV and CDR of 18F-rhPSMA-7 PET/CT
In patients with PSA level ≥ 1 ng/mL DR, and PPV were 90% and 91%, respectively, resulting in a CDR of 82%. In patients with PSA level < 1 ng/mL, DR and PPV were 69% and 85%, respectively, resulting in a CDR of 59% (Table 3).
CDR Stratified by PSA Level in Composite-Validated Subgroup
Discordant Results Between 18F-rhPSMA-7 PET Positivity and Validation
From a total of 23 PET-positive lesions in the histopathology-validated cohort, 3 lesions (13%) were confirmed as non–PCa-related. These comprised 1 lesion in the prostate bed and 2 bone lesions. A 18F-rhPSMA-7 PET-positive, but biopsy proven–negative bone lesion is shown in Figure 3. Eleven lesions were false-positive by imaging follow-up. These lesions were found in pelvic lymph nodes (6/11 regions, 55%), bone (1/11 regions, 9%), prostate bed (2/11 regions, 18%), and visceral organs (2/11 regions, 18%), as detailed in Supplemental Tables 2, 3, and 4. Eight lesions were nonevaluable (neither true- nor false-positive) and were excluded from the analysis (details in Supplemental Tables 5 and 6).
False-positive bone lesion on 18F-rhPSMA-7 PET validated by histopathology. A 69-y-old man with BCR of PCa, Gleason score 7, post-RP with follow-up PSA value of 0.37 ng/mL. Sagittal CT (A) revealed sclerotic lesion at vertebral body and posterior element of C7, showing high 18F-rhPSMA-7 uptake on sagittal 18F-rhPSMA-7 PET (B) and sagittal fused PET/CT (C) with SUVmax of 22.5. This lesion showed low signal intensity (SI) on sagittal T1-weighted MR image (MRI) (D), heterogeneous intermediate SI on sagittal T2-weighted MRI (E), with preservation of fatty marrow signal on sagittal fat-suppressed T1-weighted MRI (F). This pattern of signal intensity alteration probably corresponds to presence of hypervascularity and edema seen in early mixed active Paget disease. Biopsy at C7 was performed. Histopathologic specimen revealed reactive tissue without any PCa cells. Thorough workup of the biopsy sample definitely excluded a PCa metastasis but final diagnosis above general description of reactive tissue could not be established. Please note, that the biopsy may have the limitation in sampling errors, immunohistochemistry was not performed in this case, and it was decided to omit a rebiopsy given the unnecessary risk for patient.
PET Disease Extent According to miTNM Staging
The miTNM stage by 18F-rhPSMA-7 PET/CT is shown in Table 4. Localized disease (TrN0M0, T0N1M0, TrN1M0) was present in 43% of patients, who could be possible candidates for salvage treatment. M1 disease was present in 37% of patients. These patients evenly presented with or without locoregional disease (Tr/N1). Only a minority of these patients had a single type of distant metastases (M1a vs. M1b vs. M1c). Distribution of different miTNM stages was substantially different in the composite cohort (less Tr, more any M1 disease) given the approach to how lesions were validated.
miTNM Stage by 18F-rhPSMA-7 PET/CT
Correlation of Lesion Detection Efficacy with Clinical Parameters
DR significantly increased with PSA value, with a DR of 58%, 87%, 85%, 93%, and 95% for PSA values of < 0.5 ng/mL, 0.5 ≤ 1.0 ng/mL, 1.0 ≤ 2.0 ng/mL, 2.0 ≤ 5.0 ng/mL, and ≥5.0 ng/mL, respectively (Fig. 4). 18F-rhPSMA-7 PET/CT positivity significantly correlated with a post-RP PSA nadir of ≥ 0.1 ng/mL (P = 0.005). In contrast, PSA doubling time (dtPSA), Gleason score, TNM staging, and time from initial therapy to PSMA PET did not significantly correlate with PET DR, as shown in Table 5.
Detection rate stratified by PSA levels.
Detection Rate Stratified by PSA Level and Other Parameters on a Per-Patient Basis
In the subgroup of patients (n = 166) with very low PSA (<0.5 ng/mL), 42% of the patients had a negative scan result. PSMA PET–positive lesions in this subgroup were mainly located exclusively in the prostate bed (45%). In the subgroup of patients (n = 206) with low PSA (PSA value from 0.5 to < 2.0 ng/mL), 47% of these patients had either local or regional pelvic node recurrence, whereas about one third of them (26% for PSA 0.5 to < 1.0 ng/mL, and 31% for PSA 1.0 to < 2.0 ng/mL) had recurrence in multiple regions.
The proportion of patients who had recurrence in multiple regions increased with rising PSA value, and notably when the PSA value increased above 2 ng/mL. The percentage of recurrence in multiple regions was 13%, 26%, 31%, 62%, and 69% for PSA values of <0.5 ng/mL, 0.5 ≤ 1.0 ng/mL, 1.0 ≤ 2.0 ng/mL, 2.0 ≤ 5.0 ng/mL, and ≥5.0 ng/mL, accordingly.
DISCUSSION
Our retrospective study aimed at exploring the PPV and CDR of 18F-rhPSMA-7 PET/CT for the detection and localization of BCR in a large homogeneous population of patients after RP with a focus on lesion validation by histopathology or a composite standard of truth. Our results indicate that—similar to other PSMA-ligands for PET imaging—18F-rhPSMA-7 is effective in detecting tumor lesions even at a low PSA value (14). In more than half of the patients, lesions could be detected even at low PSA values < 0.5 ng/mL.
The PPV of 18F-rhPSMA-7 in a histopathology-validated cohort was similarly high (91%) when compared with other PSMA PET studies (ranges from 79% to 100%), summarized in a metaanalysis (6). However, composite validation delivered lower rates, indicating specific challenges especially for validation by follow-up imaging (e.g., insufficient size changes, lack of morphologic correlates from PSMA-ligand–positive lesions). The PPV of 88% confirmed by composite validation in our analysis was similar to data from a recent prospective phase III bicentric trial for 68Ga‐PSMA‐11 (15) and early data reported for the CONDOR trial using 18F-DCFPyL (84.8%–87.0% for 3 masked independent readers) (12,16). In principle, it is difficult to compare the PPV across different studies because of the different approaches used to validate lesions in the absence of histopathologic validation.
The CDR is a new term that aims to represent the detection efficacy of 18F-rhPSMA-7 PET that was proven as true-positive by composite validation. To our knowledge, this retrospective study represents the first work to use CDR as an outcome parameter. The estimated CDR varied according to patients’ PSA levels, ranging from 59% in patients with low PSA (<1 ng/mL), to 82% in patients with PSA ≥ 1 ng/mL. The results demonstrate that with both a lower DR and a lower PPV in lower PSA values, CDR as an outcome measurement currently investigated in phase III trials needs to be clearly adjusted to the patient population (CONDOR trial NCT03739684, and SPOTLIGHT trial NCT04186845).
The criteria and method of lesion validation play an important role in the CDR evaluation. In our study, most false-positive lesions validated by imaging follow-up were pelvic lymph nodes. Misinterpretation during imaging follow-up could occur for several reasons—most likely slow growth of recurrent tumor not fulfilling the prespecified validation criteria. False-positive lesions could be related to ganglia mimicking lymph nodes or PSMA-ligand uptake in reactive nodes (17,18). PSMA PET–positive bone lesions (designated as nonevaluable lesion on the basis of the applied criteria) are another source of difficult validation.
The DR of 18F-rhPSMA-7 PET/CT increases with higher strata of PSA, similar to other PSMA PET tracers (6,8–10). 18F-rhPSMA-7 has potential benefit over 68Ga‐PSMA tracers for restaging in patients with low PSA range (0.5 to < 1.0 ng/mL) as shown by the higher DR of 18F-rhPSMA-7 compared with 68Ga‐PSMA studies (87% vs. 53% to 73%) (5,6,8,19–21), with comparably excellent results in patients with PSA values > 2 ng/mL. In our study, the PSA nadir significantly correlated with PSMA positivity, whereas the dtPSA did not. This is, for example, contrary to recent results for 68Ga-PSMA-11 reported by Ceci et al. (22). Nevertheless, comparison between studies has several limitations such as the differing protocol and injected tracer activity. Thus, limited conclusions can be drawn.
A major limitation of the present work is related to the fact that no all-embracing composition validation was possible for all patients, given this study’s retrospective nature. Thus, we can only report an estimate of the CDR. A future prospective study with at least 1 lesion validation method for all patients is recommended.
CONCLUSION
18F-rhPSMA-7 PET/CT offers high PPV in BCR after RP even in low PSA values. Its CDR is dependent on the prescan PSA value, with excellent CDR in patients with PSA ≥ 1 ng/mL, which is also related to limitations validating lesions in early recurrence.
DISCLOSURE
Matthias Eiber, Hans-Juergen Wester, and Alexander Wurzer have a patent application for rhPSMA. Matthias Eiber and Wolfgang Weber are consultants for Blue Earth Diagnostics (licensee for rhPSMA). Hans-Juergen Wester is founder, shareholder, and advisor board member of Scintomics GmbH, Fuerstenfeldbruck, Germany. No other potential conflict of interest relevant to this article was reported.
KEY POINTS
QUESTION: What is the overall DR, CDR, and PPV of 18F-rhPSMA-7 PET/CT in BCR of PCa validated by composite reference standard?
PERTINENT FINDINGS: This large cohort study revealed the overall DR of 80%. The PPV of 18F-rhPSMA-7 PET/CT in composite and histopathology-proven cohorts was 88% and 91%, respectively. Thus, the CDRs in both subgroups were 70% and 73%, accordingly. There were higher DRs (90% vs. 69%), PPVs (91% vs.85%), and CDRs (82% vs. 59%) in the subgroup of patients with PSA level ≥ 1 ng/mL than in patients with PSA < 1 ng/mL. There was a significant positive correlation between 18F-rhPSMA-7 PET/CT detection efficacy and stratified PSA levels (P = 0.005), as well as PSA nadir after prostatectomy (P < 0.001).
IMPLICATIONS FOR PATIENT CARE: 18F-rhPSMA-7 PET/CT offers high PPV in BCR after RP. Its CDR is dependent on the prescan PSA value, with excellent CDR in patients with a PSA level of ≥ 1 ng/mL.
Footnotes
↵* Contributed equally to this work.
Published online Nov. 13, 2020.
- © 2021 by the Society of Nuclear Medicine and Molecular Imaging.
REFERENCES
- Received for publication August 24, 2020.
- Revision received October 19, 2020.