Visual Abstract
Abstract
99mTc-tilmanocept is a novel radiopharmaceutical for sentinel lymph node (SLN) biopsy in breast cancer. Our aim was to describe results with 99mTc-tilmanocept in a heterogeneous group of breast cancer patients scheduled for SLN biopsy. Methods: Radiotracer preparation followed the manufacturer’s indications. Local protocols for SLN detection within 9 participant centers were not changed for the entire duration of the study. In total, 344 patients with T1–T4, N0–N2 breast cancer (352 lesions) were included. Superficial (intradermal or periareolar) or deep (peritumoral or intratumoral) injections were performed. The doses were adjusted depending on the scheduled time for surgery. Results: Lymphoscintigraphy was able to depict at least 1 SLN in 339 of 352 breast lesions (96.3%), and the intraoperative SLN detection rate reached 97.2%. On univariable analysis, SLN detection rates did not differ by age, clinical T or N stage, tumor location, histologic subtype, or prior neoadjuvant therapy. Lymphoscintigraphy showed higher SLN detection in patients with a normal weight (body mass index < 25) than in those who were overweight or obese (body mass index ≥ 25), at 99.2% versus 94.6%, respectively (P = 0.031). The proportion of patients with preoperative lymphoscintigraphic detection or excised SLNs was higher with superficial than deep injections. Reinjected cases were significantly lower when superficial injection was chosen first (P < 0.001). Injection site and the tumor markers human epidermal growth factor receptor 2 and estrogen receptor had an impact on preoperative SLN visualization and intraoperative localization. In 80 cases, SLN biopsy resulted in a positive lymph node. During a mean follow-up of 19 mo, no axillary recurrences were observed. Conclusion: Whatever the protocol, 99mTc-tilmanocept showed good results in a heterogeneous breast cancer population, although the best results were achieved when a superficial injection was chosen.
Sentinel lymph node (SLN) biopsy is the standard of care in regional staging of clinically node-negative breast cancer and other types of malignancies. This technique minimizes the extent of the surgery and therefore the associated morbidity (1). Several different confirmed methods for SLN mapping exist. One method relies on visual identification of lymph nodes after the administration of vital dyes or fluorescent tracers, and the most widespread form of this method is molecular imaging visualization through preoperative lymphoscintigraphy using a radiotracer (lymphatic mapping). This method has had a high success rate (>95%, when a radiotracer is used). However, sometimes the SLN technique can fail despite correct application and imaging techniques because of an unequal pathologic examination of the SLN or complete metastatic involvement of the SLN, causing the injected tracer to bypass the infiltrated node (1).
99mTc-tilmanocept, also known as Lymphoseek (Cardinal Health), is a novel radiopharmaceutical specifically designed for lymphoscintigraphy and intraoperative SLN detection. It consists of a macromolecule of multiple units of diethylenetriaminepentaacetic acid and mannose, each attached synthetically to a dextran backbone. It accumulates in lymphatic tissue by avidly binding to mannose receptors (CD206) expressed on macrophages and dendritic precursor cells within lymph nodes (2). It was approved for lymphatic mapping by the U.S. Food and Drug Administration in 2013 and by the European Medicines Agency in 2014 (3,4). The small molecular size (7-nm diameter) of 99mTc-tilmanocept permits rapid injection site clearance, and its specific targeting to CD206 mannose-binding receptors allows avid, stable binding within target nodes. The fact that 99mTc-tilmanocept uptake in lymph nodes does not depend on the particle size might offer benefits over other radiocolloids (5,6), and 99mTc-tilmanocept exhibits other advantageous properties, such as rapid clearance from the injection site, low accumulation in distal nodes, detection of sentinel nodes near the injection site, high SLN uptake, and low leakage to higher-echelon nodes (5,7).
99mTc-tilmanocept is indicated for imaging and intraoperative detection of SLNs draining a primary tumor in adults with breast cancer, melanoma, or localized squamous cell carcinoma of the oral cavity (8). In breast cancer, the established use of 99mTc-tilmanocept is for patients with N0 disease undergoing primary surgery. The recommended dose is 50 μg of 99mTc-tilmanocept, with a total tracer activity of 18.5 MBq for same-day surgery or 74 MBq for next-day surgery (doses are not adjusted for body weight) (8).
The aim of this study was to describe and analyze outcomes for using 99mTc-tilmanocept as a radiotracer for SLN biopsy in breast cancer in actual clinical practice.
MATERIALS AND METHODS
99mTc-Tilmanocept Radiopharmaceutical Preparation
The vial components of the kit are sterile, nonpyrogenic, and contain 50 μg of tilmanocept. For radiotracer preparation, the recommendation is to use only eluate from a technetium generator that was previously eluted within 8 h. To obtain the highest radiochemical purity, the kit must be reconstituted with freshly eluted 99mTc. The tilmanocept powder in the vial must not be vented during radiolabeling.
The recommended dose of 99mTc for labeling depends on whether a 1-d or a 2-d approach is intended and will range, according to the Radiopharmacy Unit in Hospital Clínic Barcelona, from 30 to 140 MBq in a 0.65-mL volume (for a 0.5-mL dose). To compensate for activity loss, consideration is made for adhesion on the walls of vial, decay correction, and dead volume. 99mTc-sodium pertechnetate and sterile sodium chloride (9 mg/mL [0.9%] solution for injection) are added to the radiolabeled product in the tilmanocept powder vial to reach the reconstituted volume of 0.65 mL. The preparation is then allowed to stand at room temperature for at least 15 min. Radiochemical purity is determined with instant thin-layer chromatography, following the manufacturer’s instructions.
99mTc-tilmanocept solution for injection is used within 6 h after reconstitution. Individual injection volumes are no more than 0.5 mL and no less than 0.1 mL. The total injection volume is no more than 1.0 mL and no less than 0.1 mL. Dilution of the product in volumes greater than 1.0 mL could affect the in vivo disposition of the product. To reach the bottom edge of the vial and completely retrieve the dose, a spinal needle 90 mm long is used, minimizing the dead volume. No clinical consequences have been observed at dose levels 3.7 times the recommended dose of 99mTc-tilmanocept in humans (4).
SLN Biopsy Procedure
Local protocols for 9 participant centers were not changed for the entire duration of the study. All patients underwent the SLN detection technique with 99mTc-tilmanocept using preoperative lymphoscintigraphy and an intraoperative γ-probe or a portable γ-camera when this latter is available.
The method of injecting the radiotracer depended on each center’s protocol. The injection could be superficial (intradermal [n = 105] or periareolar [n = 80]) or deep (peritumoral [n = 47] or intratumoral [n = 113]). In 7 cases, the injection was a combination of both superficial and deep.
The more frequently used procedure was to inject the 99mTc-tilmanocept on the day before the surgery (2-d protocol; 314 [89.2%] cases), as opposed to the same day as the surgery (1-d protocol; 38 [10.8%] cases). The dose administered was adjusted depending on the scheduled time for SLN biopsy. The median dose (excluding reinjected patients) was 92.5 MBq (interquartile range, 74–111 MBq) for the 2-d protocol and 55.87 MBq (interquartile range, 48.1–63.08 MBq) for the 1-d protocol (Table 1).
SLN Procedure: Time Protocol, Doses, and Volume Administered
After radiotracer injection, most centers obtained 2 sets of images—the first at 30 min (early images) and the second at 2–4 h (late images). In some cases, later images (a third set of images at >6h after injection) were acquired. A single- or dual-head γ-camera with large-field-of-view detectors was generally used to acquire planar emission images (3–5 min each). A 57Co or 99mTc flood source or a 57Co or 99mTc point source was used to delineate the patient’s body contour during scintigraphy. A SPECT/CT acquisition was not mandatory and was left to the nuclear medicine physician’s discretion. When lymphatic drainage was not seen on the late images, a second dose of 99mTc-tilmanocept was administered or a different lymphatic mapping tracer, 99mTc-nanocolloid, was administered.
SLNs were defined as every node with a direct lymphatic channel from the tumor site. When the channel was not visualized, the first lymph node that appeared on scintigraphy was assumed to be an SLN. After the images were assessed by the nuclear medicine physician, a small spot of indelible ink on the skin was used to mark the SLN location.
During surgery, a handheld γ-probe or portable γ-camera was used to locate the exact positions of the SLNs. Once all SLNs were removed, they were evaluated using the probe ex vivo to demonstrate radioactivity. The wound site was checked for remaining activity. After γ-probe–guided SLN retrieval, the open axilla was palpated and suggestive lymph nodes harvested even if they did not present radioactivity.
All excised SLNs and non-SLN specimens were sent for pathologic examination. There are several different examination protocols reported in the literature, and each center used the protocols that best fit its capacity. Hence, in several centers SLNs were assessed intraoperatively using imprint cytology, frozen sectioning, or both, as well as being more thoroughly assessed after the operation. Other centers used a molecular method based on a 1-step nucleic acid amplification.
Study Design and Data Collection
This study was a retrospective analysis including 9 different Spanish centers in which breast cancer patients were scheduled for SLN biopsy. All scenarios in which SLN biopsy is currently clinically applied in breast cancer were included (e.g., primary breast cancer without clinical node involvement, breast cancer recurrence after neoadjuvant chemotherapy [with or without node involvement], male patients, and patients after previous surgery). To ensure that using 99mTc-tilmanocept for SLN biopsy in everyday routine would not affect the results obtained, every center was free to schedule patients for SLN biopsy in consensus with its own breast committee.
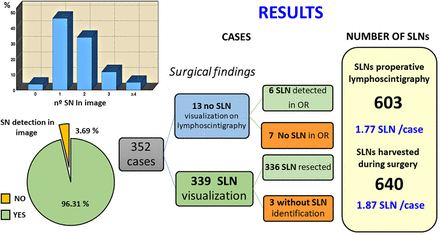
Data concerning patient and tumor characteristics, as well as the approach used, were collected. In total, 344 patients (99.1% women, n = 341) with T1–T4, N0–N2 breast cancer were enrolled from March to October 2018. Of the 344 patients, 45 had received previous neoadjuvant chemotherapy and 8 had bilateral tumors; the total number of breast lesions assessed was thus 352 (Fig. 1). The Institutional Review Board approved this study, and all patients gave written informed consent.
Patient and breast lesion distribution. Total number of unilateral lesions = 336 (97.7%); total number of bilateral lesions = 8 (2.3%).
It was anticipated that a few patients would not have an SLN seen on lymphoscintigraphy or biopsy; therefore, we wanted to examine the factors impacting failure to identify an SLN both preoperatively (lymphatic mapping findings) and intraoperatively (biopsy findings). To quantify the success of the technique, we determined several parameters. We determined the lymphoscintigraphy detection rate (LDR) and the biopsy detection rate (BDR) as the proportion of patients with at least 1 SLN lymphoscintigraphically identified or intraoperatively harvested, respectively, among all the breast lesions included. We determined the sensitivity of the SLN procedure as the percentage of patients with a positive SLN divided by all patients with positive lymph nodes (9). We defined the false-negative rate as the percentage of patients in which the SLN results were negative although the patient had positive lymph nodes on regional axillary lymphadenectomy or during the follow-up period (10) (given that lymphadenectomy was not performed on most patients and that the follow-up period was short [until March 2020], we decided to base the false-negative rate on clinical follow-up also). Finally, we determined the false-negative rate as the percentage of positive lymph nodes that were not radioactive.
Statistical analysis was done with SPSS, version 25.0. Differences were considered significant when the P value was less than 0.05.
RESULTS
Table 1 shows the protocols, the reinjections, and the doses administered. Table 2 shows the patient and tumor characteristics. Of the 344 patients, 247 (71.8%) were at least 50 y old (the 3 men were included in this group). Patients younger than 50 y had a lower body mass index (BMI) (Table 2). There were no statistically significant differences between these 2 age groups regarding pathologic findings or tumor characteristics, except for Ki-67 which was higher in the younger group (P = 0.04, Mann–Whitney U test).
Patient (n = 344) and Breast Tumor Characteristics (n = 352)
After the 99mTc-tilmanocept injection, regional lymphatic drainage was not visualized in 35 cases, and 33 of these were reinjected. After the second dose, lymphatic drainage was visualized in 22 cases (66.7%) but not in the other 11 (33.3%). Thirteen of the reinjections were with 99mTc-tilmanocept, and the remaining 20 were with a 99mTc-labeled nanocolloidal tracer. Success was greater with 99mTc-tilmanocept than with the nanocolloidal tracer, at 76.9% (10/13) versus 60% (12/20), respectively. However, the difference was not statistically significant (P = 0.314, χ2 test). In summary, lymphoscintigraphy depicted at least 1 SLN in 339 of 352 breast lesions (total LDR, 96.3%; 99mTc-tilmanocept LDR, 92.9%), and BDR reached 97.2% (Fig. 2). On univariable analysis, SLN detection rates did not differ by age, clinical T stage, clinical N stage, tumor location, histologic subtype, or prior neoadjuvant therapy (Table 3).
Distribution of lymphoscintigraphic and SLN biopsy outcomes. One patient did not undergo biopsy (patient was receiving chemotherapy). Of 352 breast lesions studied, SLN was harvested for 342, SLN was not detected for 9, and 1 patient did not undergo biopsy (was receiving chemotherapy).
Univariable Analysis Results for SLN Detection Preoperatively and Intraoperatively
Extraaxillary SLNs (inner mammary chain and intramammary) were depicted in 29 cases (8%), and their detection was related to the type of injection (superficial, 0%–4% LDR, vs. deep, 11%–14.2% LDR). Management of these SLNs differed depending on the center. Five centers did not pursue any extraaxillary nodes when these nodes were depicted by preoperative lymphoscintigraphy. The remaining 4 centers tried to perform the biopsy and obtained an SLN in 75% of extraaxillary cases. Four extraaxillary cases depicted inner mammary chain SLNs only (without axillary tracer uptake), and it was possible to retrieve an SLN from the inner mammary chain in 3 cases.
Regarding the 45 women who had previously received neoadjuvant chemotherapy, the χ2 test did not show statistical differences in LDR (P = 0.257), BDR (P = 0.098), or positive pathology results (P = 0.082) from patients without neoadjuvant therapy. The percentage of SLN detection by preoperative lymphoscintigraphy in patients with a normal body weight (BMI < 25) was significantly higher than in those who were overweight or obese (BMI ≥ 25) (LDR, 99.2% vs. 94.6%, respectively; P = 0.031); however, BMI did not affect BDR (P = 0.325).
The variables that affected both LDR and BDR were site of injection and the tumor markers human epidermal growth factor receptor 2 (HER-2) and estrogen receptor. Regarding tumor HER-2 and estrogen receptor markers, the data showed a relationship between patients with HER-2 negativity or ER positivity and better detection rates. Considering only the cases with the luminal or HER-2 molecular subtype (thus excluding basallike tumors), univariable analysis indicated that a higher proportion of luminal cancers achieve satisfactory results, with significantly better LDR and BDR (luminal vs. HER-2 molecular subtype: LDR = 97.1% vs. 88.2%, P = 0.012; BDR = 98.5% vs. 85.3%, P < 0.001).
Both LDR and BDR were higher when superficial administration was used instead of deep administration (LDR = 98.8% vs. 93.8%, P = 0.009; BDR = 99.5% vs. 95%, P = 0.010). On the other hand, the reinjected cases were significantly lower when superficial injection was chosen than when deep injection was chosen (P < 0.001, χ2 test).
The number of SLNs detected by preoperative lymphoscintigraphy was 603, whereas the number of intraoperatively detected SLNs was 640 (Table 4). The number of SLNs retrieved during surgery was coincident with the number depicted on lymphoscintigraphy in 239 cases. However, in 41 cases (11.65%), lymphoscintigraphy detected a higher number of SLNs than the number intraoperatively harvested, and in 72 cases (20.45%), the number of SLNs depicted on lymphoscintigraphy was lower than the number retrieved during surgery. The number of excised SLNs was 1 or 2 in 75.3% of all patients, but for breast lesions in which the biopsy was successful, that percentage increased to 77.5% (Fig. 3).
Number of SLNs Detected by Lymphoscintigraphy and Harvested
Example breast cancer patient intratumorally injected with 70 MBq of 99mTc-tilmanocept in 2-d protocol. Planar images were obtained at 30 min, 2 h, and 21 h after injection. Two well-depicted SLNs are clearly seen in all sets of images. Three-dimensional volume-rendered images, based on SPECT/CT data (right corner in upper and lower rows), accurately reflect SLM anatomic localization. BMI = body mass index; IT = intratumoral injection; LOQ = lower outer quadrant; OR: operation room (surgery).
Concerning T stage, the SLN detection rate for lymphoscintigraphy was similar among the T1–T3 groups. The SLN was successfully depicted in 81%–100% of T1–T3 tumors and in 50% of T4 tumors. Concerning intraoperative harvesting, the SLN was excised for 96.7%–100% of T1–T3 tumors and for 50% of T4 tumors. Concerning N stage, LDR was 96.4% for N0 cases, and BDR was 97.5%. LDR was 97.2% for N1 and N2 cases, and BDR was 97.2% for both. These differences were not statistically significant.
In 80 cases, the histopathologic SLN result produced a positive lymph node (Table 2), with the analysis showing 41 cases of micrometastasis and 39 of macrometastasis. Interestingly, only 1 non-SLN metastatic lymph node was identified. Therefore, the sensitivity was 98.76% and the false-negative rate was 2.3%. The percentage of positive SLNs was moderately higher in patients younger than 50 y (27%) than in those older than 50 y (21%), but this difference was without statistical significance. The overall false-negative rate after a mean follow-up of 19 mo (range, 16–25 mo) was 0% (no axillary recurrences).
DISCUSSION
Lymphatic mapping and SLN biopsy have demonstrated their accuracy for correctly staging patients with clinically negative regional nodes. Lymphoscintigraphy is the only modality ascertaining the SLN location and lymphatic drainage. The main aim of lymphoscintigraphy is to provide a visual road map of nodal basins at risk before surgery, showing the orderly progression of lymphatic flow reaching the lymph node (11). Lymphoscintigraphy for SLN mapping must be reproducible, and this issue has been assessed in various malignancies (12–14). There is a large geographic variability in radiotracer availability; 99mTc-nanocolloids ranging from 15 to 100 nm are the most widely used in Europe, whereas filtered and unfiltered 99mTc-sulfur colloid (particle size, 20–1,000 nm) is usually applied in the United States (15).
The evolving oncologic multidisciplinary approach has generated new indications for SLN mapping (especially in breast cancer) that result in controversial issues and protocol differences among different researchers (16). Against this background, 99mTc-tilmanocept, a new radiotracer designed specifically for the identification of SLNs, has become available in the United States and Europe. This tracer has distinct properties for SLN identification and lymphatic mapping, with the potential to overcome the shortcomings of the previously used radiotracers for SLN biopsy (17). Some studies showed 99mTc-tilmanocept to be superior to filtered 99mTc-sulfur colloid and vital dyes—a superiority that is probably based on properties such as quick clearance from the injection site and high uptake in first-tier lymph nodes during the first hour, leading to an adequate number of excised SLNs per patient.
Some studies have compared filtered 99mTc-sulfur colloid with 99mTc-tilmanocept. Baker et al. reported a similar SLN detection rate and excision of fewer SLNs in patients injected with 99mTc-tilmanocept (mean, 1.85 SLNs) than in patients for whom filtered 99mTc-sulfur colloid was used (mean, 3.24 SLNs) (18). These results are concordant with those of the present study, in which the mean number of harvested SLNs was 1.82.
Wallace et al. reported results from a trial of 13 different centers that included 148 patients (19). Each patient received 99mTc-tilmanocept and a vital blue dye. The primary endpoint was the proportion of SLNs detected by blue dye and radiotracer. During surgery, 207 of 209 SLNs located by blue dye were also located by 99mTc-tilmanocept (concordance rate, 99%). However, the radiotracer intraoperatively showed 320 SLNs and identified at least 1 SLN in 146 patients, and blue dye identified SLNs in 131 patients. 99mTc-tilmanocept accurately detected 31 of 33 positive SLNs, whereas blue dye detected only 25 of these 33 nodes (19). In our protocol, blue dye was not specifically used, but 99mTc-tilmanocept accurately identified 80 of 81 positive SLNs (similar to the study of Wallace et al.).
Unkart et al. reported that performance results in 617 patients after a single intradermal injection of 99mTc-tilmanocept did not differ significantly between the 1-d and the 2-d protocols (20). The prolonged time provided by the 2-d protocol showed that SLN accumulation of 99mTc-tilmanocept can persist for at least 24 h after administration (20). In our study, a 2-d approach was used for 314 (89.2%) patients, but in only 3 patients could an SLN not be intraoperatively found after a lymph node had been well depicted on preoperative lymphoscintigraphy.
On the other hand, we found statistically significant differences in LRD and BRD between superficial and deep injections of radiotracer. This fact was previously reported by Hellingman et al. in a large study (2,050 patients) using a deep intratumoral injection of nanocolloids (a method that results in a higher percentage of visualization of extraaxillary drainage). Preoperatively, the SLNs were visualized on lymphoscintigraphy in 86.7% of the procedures (93.8% in our group with the same injection method) and lymphatic drainage was not visualized in 13.3% (273/2,050). These authors recommended use of a superficial periareolar injection in patients for whom the prognostic and therapeutic relevance of inner-mammary-chain SLN identification is limited (21). Like the study of Hellingman et al., the present study showed higher extraaxillary drainage, ranging from 11% to 14.2% when deep tracer injection was performed.
Tokin et al. retrospectively compared 99mTc-tilmanocept with 99mTc-nanocolloid (the most frequently used radiotracer in Europe) (22). Six studies were included in a metaanalysis, and 5 of them, involving 6,134 patients, were used to calculate the 99mTc-nanocolloid SLN localization rate (mean, 95.9%). 99mTc-tilmanocept was used in 148 patients, and pooled analysis revealed a mean localization rate of 99.9%, showing its superiority in localization rate (22). A comparison of the 2 radiotracers was beyond the scope of the present study, but the BDR in our study was 97.2% (342/352), slightly higher than the results of this metaanalysis with 99mTc-nanocolloid. There are, however, some concerns to be addressed. A direct head-to-head comparison with the standard radiotracer used in Europe has not been done. Such a comparison must be completed to ascertain whether there are no or minimal variations through time with this new tracer.
In our study, patient age did not affect either pathology results or tumor characteristics, except for the Ki-67 marker. Patients older than 50 y had higher BMIs, and a BMI of more than 25 affected the LDR; therefore, patients who are overweight or obese could have lymphoscintigraphy results that are difficult to interpret. However, because BMI does not affect BDR, obese patients are good candidates for SLN biopsy.
SPECT/CT is usually performed when lymphoscintigraphy in cancer patients does not show the SLN (especially with the new indications concerning recurrent surgery, previous radiotherapy, or neoadjuvant chemotherapy) or when there are difficult-to-evaluate nodes (e.g., in obese patients with faint SLN uptake). It is well known that SPECT/CT improves SLN detection for several types of tumor, especially in obese patients (23,24). Pouw et al. showed that SPECT/CT was able to clearly depict an SLN in 23% of patients for whom an SLN could not be visualized on planar scintigraphy (25). SPECT/CT was not mandatory in the present study and was left to clinical judgment of the nuclear medicine physician. The use of SPECT/CT might have increased the positive detection results in our study, but we decided to perform the basic approach (planar imaging) at all centers.
In the same study of Pouw et al., reinjection of the tracer achieved 62% SLN visualization in those cases with persistent negative results for SPECT/CT visualization. In our study, after reinjection of 33 patients whose planar images had been negative for an SLN, 22 cases succeeded in visualizing an SLN. When reinjection was used, 99mTc-tilmanocept resulted in a higher percentage of visualization than did 99mTc-nanocolloid (76.9% vs. 60%).
Although the established use for 99mTc-tilmanocept is in patients with N0 disease undergoing primary surgery, our study used different patient profiles and the data showed no statistical differences in LDR, BDR, or pathology results between different N or T stages, even in patients with prior neoadjuvant chemotherapy. It is remarkable to note that despite the small number of patients who received such therapy, these patients were as good a candidate for 99mTc-tilmanocept mapping as patients who did not receive prior neoadjuvant therapy; no differences in detection rates were found between the two groups.
The recommended dose of 99mTc-tilmanocept is 50 μm in 18.5 MBq for same-day surgery or 74 MBq for next-day surgery. In the present study, higher doses did not show adverse effects. Additionally, reinjection resolved the no-drainage situation most of the time. Therefore, the option of using a higher dose without adverse events could be an advantage in actual clinical practice. Some patients received 100 μg of 99mTc-tilmanocept, but no side effects were recorded.
99mTc-tilmanocept offers good results regardless of patient type or tumor characteristics; the factors that affected the SLN biopsy results were the injection site and the molecular subtype. In this respect, an intradermal or subareolar injection site resulted in better LDR and BDR and were most advantageous in patients with luminal tumors. There is a tendency to use a superficial tracer injection in patients considered at low risk for lymph node metastases, whereas in high-risk patients, with large or multifocal tumors or lesions located deep or mediocaudally in the breast, deep tumor-related injections are recommended in order to stage as accurately as possible both intraaxillary and extraaxillary lymph nodes. An alternative is to use both deep and superficial injections simultaneously (26). We found statistically significant differences in SLN visualization and excision rates between superficial and deep injections, such that use of superficial injections (or of superficial injections combined with deep ones) might be warranted, but further study is required before more specific recommendations can be made.
In medical practice today, costs are important. The cost of one vial of tilmanocept is higher than the cost of the most used tracer for lymphatic mapping. The reported advantages of 99mTc-tilmanocept versus the radiocolloid tracers include faster clearance from the site and higher retention in the SLN (avoiding extensive node dissection and morbidity). Other potential benefits could be the reduced number of SNs to be assessed by the pathologist (time reduction), with similar staging results. However, a head-to-head comparison of 99mTc-tilmanocept with standard SLN radiotracers in a larger series of patients is necessary.
Finally, 99mTc-tilmanocept was able to demonstrate metastatic involvement (macro- or micrometastasis) in 22.7% of patients. When a positive result in an SLN was obtained, the next therapeutic step was left up to the local breast committee. Most centers applied the criteria of the American College of Surgeons Oncology Group Z0011 trial when conservative breast surgery was used (avoiding lymphadenectomy in most of cases, even those with a positive SLN). When a positive SLN was observed after neoadjuvant chemotherapy (macro- or micrometastasis), a complete axillary lymphadenectomy was performed. On the other hand, after surgery, all decisions on systemic adjuvant treatment and radiotherapy were based on international guidelines (European Society for Medical Oncology, European Society for Radiotherapy and Oncology, and National Comprehensive Cancer Network). In the present study, after a mean follow-up of 19 mo, the false-negative rate was 0%. The results are similar to those obtained in previous studies (11,18,19) with the same radiotracer, confirming its consistency throughout different approaches and clinical situations.
CONCLUSION
Whatever the protocol, 99mTc-tilmanocept showed good results in a heterogeneous breast cancer population, although the best results were achieved when a superficial injection was chosen.
DISCLOSURE
No potential conflict of interest relevant to this article was reported.
ACKNOWLEDGMENT
We thank Ana Villarejo López for help with statistical analysis and for her dedication and collaboration throughout this work.
KEY POINTS
QUESTION: What are the outcomes in a real clinical practice of using 99mTc-tilmanocept for SLN biopsy in breast cancer?
PERTINENT FINDINGS: 99mTc-tilmanocept tracer is able to precisely localize the SLN whatever protocol is used, although the best results are achieved when a superficial radiotracer injection is performed.
IMPLICATIONS FOR PATIENT CARE: The results foster the clinical reliability of the technique using this novel tracer.
Footnotes
Published online Oct. 9, 2020.
- © 2021 by the Society of Nuclear Medicine and Molecular Imaging.
REFERENCES
- Received for publication June 21, 2020.
- Accepted for publication September 15, 2020.