Abstract
1119
Objectives: We have recently reported the synthesis of [11C]deferiprone, a novel imaging agent to be used for the assessment of iron dyshomeostasis.1 Initial radiolabeling attempts of [11C]deferiprone proved difficult due to unforeseen complications such as radiolysis and the arduous task of purification of the compound from its precursor. Purification of the compound has remained elusive due to the close retention times of the product and deprotected N-desmethyl precursor on many semipreparative HPLC column types: HILIC, and Reverse Phase (RP) stationary phases. The optimization of the synthesis of [11C]deferiprone to provide a high-yielding and stable product is of high interest give the compounds FDA approval, ability to chelate ferric iron (Fe3+), and concurrent use in Alzheimer’s Disease Phase 2 clinical trials.2 Fe3+ has been found to accumulate abnormally within neurons leading to an oxidatively stressed environment, resulting in inflammation and neuronal loss of various brain structures such as the Substantia Nigra or Motor Cortex, of which correspond to either Parkinson’s Disease (PD) or Amyotrophic Lateral Sclerosis (ALS), respectively.3 This method of neurodegeneration has been coined as the Metal Hypothesis of Neurodegeneration of which we seek to probe with the use of metal chelating radiotracers.4 Currently, there are no radiotracers available for the imaging of ALS, and those available for PD largely image the loss of neuronal density as opposed to the accumulation of aggregates. Therefore, the objective of this study is to method to produce doses of [11C]deferiprone to be used for the assessment of multiple neurodegenerative diseases in preclinical and clinical studies.
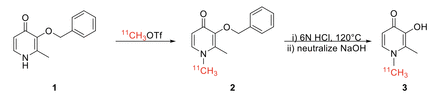
Methods: [11C]Deferirpone was synthesized from a benzyl protected N-desmethyl precursor (1) using an automated radiochemistry synthesis module. [11C]CH3OTf was produced by standard methods from cyclotron produced [11C]CO2. The precursor (1, 1mg) dissolved in DMF (100 μL) was sparged with [11C]CH3OTf for 3 min at room temperature. Deprotection of the benzyl group was accomplished in 3 min with 6N HCl (500 µL) and ascorbic acid (10 mg) at 120°C. The reaction mixture was cooled and quenched with 12N NaOH (300µL) and purified by quantitative HPLC (SYNERGI Hydro-RP 10μ 250x4.6 mm) at a flow rate of 2 mL/min. The product peak (rt~6 min.) was collected for 1.5 minutes, yielding 1.8 mCi of product.
Results: Methylation of the benzyl protected [11C]deferiprone precursor was assessed and observed to be synthesized in 17% radiochemical yield (RCY, 33.8 mCi from 200 mCi of [11C]CH3I) confirmed by analytical HPLC (SYNERGI Hydro-RP 4µ 250x4.6mm) co-injection with unlabeled reference standard. Multiple deprotection conditions were tested both manually and in an automated setup, with 6N HCl giving full deprotection in 5.2% RCY (25.6 mCi from 500 mCi of [11C]CH3I). To halt radiolysis, a mobile phase with ascorbic acid was utilized (2% EtOH 10 mM NaOAC, 1mM Ascorbic Acid pH 3.77). Furthermore, switching from a 4µ semipreparative Hydro-RP column to a 10µ analytical column has made it possible to purify the product without the presence of precursor in the final dose.
Conclusions: The synthesis and purification of [11C]deferiprone for use in studies have been developed. Radiolytic decomposition has been stopped through the use of ascorbic acid in both the deprotection conditions and HPLC buffer during purification. References: 1. Tanzey, S.S.; Brooks. A. F.; Shao X.; Scott. P.J.H. J Nucl Med, 2019 vol. 60 no. 1 16212. Martin-Bastida, A.; Ward, R. J.; Newbould, R..; et al. Sci. Rep. 2017, 7 (1), 1398.3. Singh, N.; Haldar, S.; Tripathi, A. K.; et al. Antioxid. Redox Signal. 2014, 20 (8), 1324-1363. 4. Bush AI; Tanzi RE. Neurotherapeutics. 2008, 5( 3), 421-32