Abstract
1027
Introduction: Precision oncology treatment requires personalized interventions to meet the needs of patients. Immune checkpoint inhibitors (ICIs) is a revolutionary example of immunotherapy that are changing the clinical management of cancer. However, the response rate of different patients to immunotherapy varies greatly. Therefore, an accurate and repeatable imaging method is urgently needed to determine the number of patients who are most likely or least likely to respond to immunotherapy. Positron emission tomography (PET) has been widely used in clinical tumor staging and efficacy monitoring. Optical imaging can supplement PET, for example, as an auxiliary means of tumor resection. This dual label imaging agent is especially useful in a clinical program that uses non-invasive detection of PD-L1 expression by initial PET scan, followed by near infrared fluorescence (NIRF) imaging to guide tumor resection. In this study, we labeled anti-PD-L1 antibody with 89Zr and NIRF dyes for dual-mode PET / NIRF imaging of PD-L1 expression in mouse tumors.
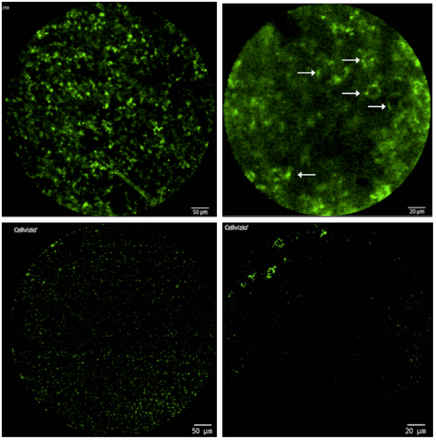
Methods: DFO and IRDye800cw were conjugated to anti-PD-L1 and labeled with 89Zr. Flow cytometry and confocal microscopy were used to screen tumor cells with high and low expression of PD-L1. 6-8 weeks old male C57BL/6 mice were subcutaneously injected with 1 × 106MC38 cells suspended in 100 µL PBS.When the tumors reached ~500 mm3, mice were intravenously (i.v.) injected with 89Zr-DFO-PD-L1-IRDye800cw(3.7±0.1MBq) and imaged with PET at 2, 12, 24, 48, 72, and 96h. 6-8 weeks old male BALB/c nude mice were implanted subcutaneously in opposite sides of shoulders with 106of MC38 cells suspended in 100 µL PBS( right shoulder) and CT26 cells suspended in 100 µL PBS( left shoulder). NIFR was carried out to examine the biodistribution and tumor-targeting effects of 89Zr-DFO-PD-L1-IRDye800cwin vivo, imaged at 2, 12, 24, 48, 72, and 96h. Major organs were subjected for ex vivo NIRF imaging at 96h. In vivo CLE was performed on the MC38 tumor and CT26 tumor models using a probe-based CLE system (Cellvizio® Mauna Kea Technologies), 24h after FITC-PD-L1injection. Anti-PD-L1 was applied to MC38 and CT26 tumors to verify the different response of tumors to immunotherapy at different levels of PD-L1 expression.
Results: It was found that the high expression of PD-L1 in MC38 tumor cells and the low expression of PD-L1 in CT26 tumor cells.PET imaging of C57BL/6 mice( with MC38 colorectal tumor) showed that tumor uptake could be seen after 24h i.v. 89Zr-DFO-PD-L1-IRDye800cw and then increased gradually with time, peaked in 72h, and then decreased gradually, while liver uptake could be seen at 12h after injection and then increased gradually with time. For the NIFR imaging, the uptake of MC38 tumor( right shoulder) is visible, while CT26 tumor ( left shoulder) is almost non uptake. This result is similar to the level of PD-L1 expressed in cells detected by flow cytometry. It is confirmed that 89Zr-DFO-PD-L1-IRDye800cw can detect the expression of PD-L1 noninvasively.The CLE results showed that a specific PD-L1-positive fluorescence signal was present in the MC38 neoplastic region, no obvious fluorescence signal was detected in CT26 neoplastic regions.
Conclusions: Herein we report PET/NIRF imaging of PD-L1 expression using 89Zr-DFO-PD-L1-IRDye800cw, noninvasive detection of PD-L1 expression in different tumors, guided surgical resection and evaluation of treatment response.