Abstract
The effects of tobacco smoking on the immune system of the brain are not well elucidated. Although nicotine is immunosuppressive, other constituents in tobacco smoke have inflammatory effects. PET imaging of the 18-kDa translocator protein (TSPO) provides a biomarker for microglia, the primary immunocompetent cells of the brain. This work compared brain TSPO levels in 20 tobacco smokers (abstinent for at least 2 h) and 20 nonsmokers using a fully quantitative modeling approach for the first time, to our knowledge. Methods: 11C-PBR28 (N-((2-(methoxy-11C)-phenyl)methyl)-N-(6-phenoxy-3-pyridinyl)acetamide) PET scans were acquired with arterial blood sampling to estimate the metabolite-corrected input function. 11C-PBR28 volumes of distribution were estimated throughout the brain with multilinear analysis. Results: Statistical analyses revealed no evidence of significant differences in regional 11C-PBR28 volumes of distribution between smokers and nonsmokers (whole-brain Cohen d = 0.09) despite adequate power to detect medium effect sizes. Conclusion: These findings inform previous PET studies reporting lower TSPO radiotracer concentrations in the brain (measured as SUV) for tobacco smokers than for nonsmokers by demonstrating the importance of accounting for radiotracer concentrations in plasma. These findings suggest that nonsmokers and smokers have comparable TSPO levels in the brain. Additional work with other biomarkers is needed to fully characterize the effects of tobacco smoking on the brain immune system.
Tobacco smoking is a leading cause of preventable disease and death, annually responsible for 6 million deaths (1). Many negative health consequences associated with tobacco smoking, including cancer, respiratory infection, and bacterial meningitis (2), involve altered immune function. These immune impairments may be mediated by free radicals, toxic aldehydes, or other inflammatory constituents present in tobacco smoke (3). Tobacco smokers exhibit significantly higher circulatory levels of the proinflammatory proteins C-reactive protein and interleukin-6 (4,5) than nonsmokers, potentially contributing to an increased prevalence of diseases characterized by chronic inflammation (6).
Although the effects of tobacco smoking on peripheral immune signaling have yet to be fully elucidated, even less is known about the effects on the immune system of the brain. Knowledge of these effects is important because dysregulated brain immune signaling contributes to compulsive drug use and common comorbidities of drug use (7,8). Microglia, the primary immunocompetent cells of the brain, are critical to maintaining neuronal homeostasis and synaptic plasticity (9). Consequently, neuroimmune pharmacotherapies, such as minocycline and ibudilast, have generated interest for smoking cessation (10). However, tobacco smoking has complex effects on the brain. Although many constituents of tobacco smoke are proinflammatory, nicotine itself has antiinflammatory effects (11). Additionally, the blood–brain barrier likely alters smoke-constituent exposure in the brain relative to the body. Therefore, research directly examining neuroimmune markers in tobacco smokers is critical to inform approaches that might improve health outcomes.
An imaging approach to studying the immune system of the brain is PET imaging of the 18-kDa translocator protein (TSPO) (12). TSPO is expressed primarily in microglia and to a lesser extent in astrocytes and endothelial cells. The activation of microglia in response to pathogens or injury (13) increases TSPO expression in microglia. Microglial activation is a hallmark of the brain immune response and is critical for repairing cells and reestablishing homeostasis (14,15). PET radioligands with high affinity and specificity for TSPO, such as 11C-PBR28 (N-((2-(methoxy-11C)-phenyl)methyl)-N-(6-phenoxy-3-pyridinyl)acetamide) (16–18), can be used to estimate the ligand distribution volume (VT) (19), which is proportional to the number of in vivo sites available for 11C-PBR28 binding (i.e., TSPO availability). The goal of this work was to compare TSPO levels in tobacco smokers and nonsmokers using fully quantitative PET imaging. On the basis of preclinical research showing that exposure to tobacco smoke increased neuroimmune markers (20,21), we hypothesized that tobacco smokers would have higher TSPO levels than nonsmokers. Interestingly, 2 previous studies reported significantly decreased brain concentrations of a TSPO-specific radioligand in the brains of tobacco smokers compared with nonsmokers (22,23). A major limitation of these reports was the outcome measure of normalized radioligand concentrations in the brain (SUV). For all PET radiotracers, brain SUVs alone do not account for radioligand concentrations in plasma. Because the plasma input function is not known, brain SUV may be dissociated from target protein levels (such as TSPO). Thus, a secondary goal of this work was to compare fully quantitative VT measures of TSPO availability with semiquantitative SUV measures.
MATERIALS AND METHODS
Subjects
Twenty tobacco smokers (15 men and 5 women) and 20 age-matched healthy nonsmokers (13 men and 7 women) underwent an 11C-PBR28 PET scan with arterial blood sampling and an anatomic MRI scan. Before scanning, the subjects were genotyped for the rs6971 single-nucleotide polymorphism, which affects 11C-PBR28 affinity for the TSPO site (24), as previously described (25). T/T homozygotes (low-affinity binders) were excluded. Data from a subset of subjects (4 smokers and 10 nonsmokers) have been reported previously (26,27).
All procedures were approved by the Yale School of Medicine Human Investigation Committee and the Radiation Safety Committee. Written informed consent, approved by the Human Investigation Committee, was obtained from all subjects before participation in this study. All subjects underwent screening procedures that included a physical examination, electrocardiogram, routine blood tests, and urine toxicologic analysis. None had any significant findings on screening or any history of major medical disorders, and none met any of the criteria for current or past psychiatric or substance abuse diagnosis (other than nicotine dependence for cigarette smokers) as described in the 4th edition of the Diagnostic and Statistical Manual of Mental Disorders. All female participants were required to have a negative pregnancy test on the scanning day before receiving the radiotracer. Smokers were required to have smoked cigarettes daily for at least 1 y. A current smoking status was confirmed during intake by carbon monoxide levels greater than 10 parts per million (ppm) and urine cotinine levels greater than 150 ng/mL.
On the scanning day, the subjects completed a questionnaire characterizing mood, including the Beck Depression Inventory (28). Tobacco smokers completed measures of tobacco smoking behavior, including the Fagerström Test for Nicotine Dependence (29), the Minnesota Nicotine Withdrawal Scale (30), and the Urge to Smoke Questionnaire (31). Tobacco smokers were abstinent for a minimum of 2 h before scanning and a maximum of 14 h. Levels of carbon monoxide and urine cotinine, the primary metabolite of nicotine, were also collected on the scanning day.
Data Acquisition
11C-PBR28 was produced as previously described (25). PET imaging data were acquired with a High-Resolution Research Tomograph (CTI/Siemens Medical Solutions). Head motion data were acquired with an optical motion-tracking device (Vicra; NDI Systems). PET imaging scans began with acquisition of a 137Cs transmission scan over 6 min. Emission data acquisition began simultaneously with bolus injection of 540 ± 181 MBq of 11C-PBR28 and continued for 120 min. Arterial blood samples were collected during scanning to measure the metabolite-corrected input function as previously described (32). The plasma free fraction was measured in triplicate with ultrafiltration techniques as previously described (25).
MRI data were used to provide careful anatomic localization of 11C-PBR28 uptake in brain. MR data were acquired using a 3-T Siemens Trio scanner with a weighted gradient-echo sequence (echo time, 3.3 ms; inversion time, 1,100 mS; repetition time, 2,500 ms; fractional anisotropy, 7°) to result in images with 1 mm3 isotropic resolution.
Imaging Data Analysis
Dynamic list-mode emission data were histogrammed in time frames ranging from 30 s to 5 min and reconstructed with the motion-compensation ordered-subsets expectation maximization list-mode algorithm for resolution recovery, which includes event-by-event motion correction. The first 10 min of PET data were summed and registered to the subject’s anatomic MR data using a mutual information algorithm constrained to 6° of freedom (Oxford Centre for Functional Magnetic Resonance Imaging of the Brain’s Linear Image Registration Tool, Functional Magnetic Resonance Imaging of the Brain Software Library, version 3.2). The native MR image was coregistered into the Montreal Neurologic Institute template space using a nonlinear transformation algorithm (BioImage Suite). Atlas-defined bilateral regions of interest were transformed from the Automated Anatomic Labeling template space (33) to native PET space to extract 11C-PBR28 time–activity curves. Analyzed brain regions included the amygdala, hippocampus, caudate, putamen, thalamus, whole cerebellum, and frontal, occipital, parietal, and temporal cortices.
The primary outcome measure for all PET scans was 11C-PBR28 VT, the equilibrium ratio of 11C-PBR28 concentration in tissue to that in arterial plasma. Values of VT were estimated with multilinear analysis (34) (t* = 30 min) as previously validated, which yields a test–retest reproducibility of 7%–9% in our center (32).
Statistical Analysis
Student t tests were used to compare age, Beck Depression Inventory scores, and radiotracer injection parameters between smokers and nonsmokers. A linear mixed modeling approach was used for statistical analysis of 11C-PBR28 VT values. To test the null hypothesis of no difference in 11C-PBR28 VT between smokers and nonsmokers, a single model was constructed featuring rs6971 genotype as a fixed factor, smoking status as a between-subjects factor, and brain region as a within-subject factor. Post hoc linear contrasts examined regional differences in 11C-PBR28 VT between smokers and nonsmokers and were considered significant for an α value of less than 0.05 (uncorrected for multiple comparisons). As a secondary analysis, the same statistical approach was repeated using brain SUV (instead of VT), averaged 90–120 min after injection, as an outcome measure.
Exploratory analysis with linear regression examined for potential relationships between smoking behaviors (cigarette use, time of last cigarette, Fagerström Test for Nicotine Dependence, and Minnesota Nicotine Withdrawal Scale; smoking group only) or mood (Beck Depression Inventory; all subjects) with 11C-PBR28 VT values. For these analyses, rs6971 genotype was accounted for by creating a z score for each variable within each genotype group (medium-affinity binder or high-affinity binder, not separated by smoking status for Beck Depression Inventory) as previously described (35). Linear regression analysis was performed on these variables with no correction for multiple comparisons because of the exploratory nature of the data.
RESULTS
On average, tobacco smokers (n = 20) reported smoking 15 ± 8 cigarettes/d for 16 ± 9 y, with Fagerström Test for Nicotine Dependence scores of 6 ± 2, Urge to Smoke Questionnaire–Intent scores of 10 ± 4, and Urge to Smoke Questionnaire–Relief scores of 11 ± 5. These characteristics are consistent with moderate to high levels of nicotine dependence (Table 1). There were no significant group differences in age or Beck Depression Inventory score. The injected PBR28 mass was greater in nonsmokers than in smokers (P = 0.002). There were no significant group differences in injected 11C-PBR28 radioactivity dose or plasma free fraction.
Subject and 11C-PBR28 Imaging Parameters
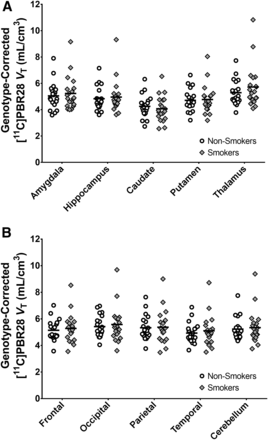
Analysis of 11C-PBR28 VT values did not reveal a significant main effect of smoking (F1,36 = 0.16, P = 0.70; effect of genotype, F1,36 = 52.88, P < 1ˣ10−8; smoking status–by–region interaction, F9,354 = 1.8, P = 0.07). Although these results indicated a trend for a smoking status–by–region interaction, post hoc linear contrasts revealed no significant differences in 11C-PBR28 VT between smokers and nonsmokers in any region. The region with the largest group difference was thalamus (F1,37 = 0.69, P = 0.41). To better characterize this null finding, we estimate a genotype-corrected whole-brain effect size (Cohen d) of 0.09, with a 95% confidence interval of −0.44 to 0.62. These findings are illustrated in Figure 1. For completeness, individual values are shown uncorrected for genotype in Supplemental Figure 1 (supplemental materials are available at http://jnm.snmjournals.org). No significant relationships between 11C-PBR28 VT and mood or smoking measures were detected.
11C-PBR28 VT values do not significantly differ between smokers (n = 20) and nonsmokers (n = 20). 11C-PBR28 VT values are adjusted for rs6971 genotype for visualization purposes by incorporating genotype coefficient estimated by mixed model.
Secondary analyses of 11C-PBR28 SUVs similarly revealed no significant main effect of smoking (F1,36 = 0.22, P = 0.64; effect of genotype, F1,32 = 7.5, P = 1 × 10−3) but a significant smoking status–by–region interaction (F9,354 = 2.45, P = 0.01). Post hoc linear contrasts did not identify significant differences in 11C-PBR28 SUVs in any regions. The region with the largest group difference was thalamus (F1,37 = 0.91, P = 0.35). These findings are illustrated in Supplemental Figure 2.
DISCUSSION
This study did not find evidence of differences in TSPO availability (measured by 11C-PBR28 VT) between tobacco smokers and nonsmokers, despite adequate power to detect moderate effect sizes. Since TSPO levels are sensitive to microglia activation, these findings suggest that microglia may not be chronically activated in tobacco smokers. Caution in this interpretation is required because TSPO levels may be affected by other factors, including altered TSPO expression in nonmicroglial cells, change in the numbers of cells expressing TSPO, or abnormal cell metabolism (36). Nonetheless, the result is not consistent with preclinical reports of increased neuronal cytokine expression and oxidative stress markers after tobacco smoke exposure (20,21). Therefore, the neuroimmune effects of tobacco smoking are likely subtler than chronic activation of the brain immune system.
The present finding is not consistent with previous TSPO PET imaging reports that tobacco smokers exhibited lower brain SUV levels at early times after injection (20–40 min) than nonsmokers (22,23). Considering that our study exhibits sufficient power to detect medium effect sizes, underlying methodologic issues likely explain the seemingly conflicting results. An interpretation that synthesizes our data with these previous reports is that smokers have a different radiotracer distribution in the periphery or differences in radiotracer influx, whereas TSPO availability is comparable between the 2 groups. Differences in radiotracer metabolism or bioavailability are a well-known phenomenon in scenarios such as exogenous drug administration (37) or disease states (38). This same mechanism could explain the reported associations of brain SUV with number of cigarettes smoked per day found using a different radiotracer, 11C-DAA1106 (39). In our sample with the radiotracer 11C-PBR28, we did not find evidence of significant group differences in brain SUV at late times (90–120 min after injection). This finding may result from differences in the kinetic properties of the 2 radioligands used (40), low levels of specific binding with 11C-DAA1106, or the use of a later time frame (chosen to reduce the influence of radiotracer delivery and flow effects on SUVs). In summary, the fully quantitative analyses performed here do not indicate differences in TSPO availability between tobacco smokers and nonsmokers.
Studies of baseline TSPO availability do not definitively address the question of the possible immunosuppressive effects of tobacco smoking on the brain. A single scan does not capture the dynamic nature of the brain immune system. Approaches that measure the TSPO response to an acute immune stimulus such as endotoxin can provide an immune stress test reflecting the capacity of neuroimmune response (i.e., microglial activation) in study populations (27,41). These studies are of high interest for future work to determine whether tobacco smoking suppresses (or exacerbates) neuroimmune response. Alternatively, newer imaging targets such as cyclooxygenase-2 (42) or reactive oxygen species (43) may provide biomarkers more directly related to tissue damage (i.e., inflammation). Such approaches may improve our understanding of the effects of tobacco smoking on brain immune signaling.
There are several limitations to this study. First, the injected PBR28 mass was significantly greater in nonsmokers than in smokers. A large injected mass can reduce outcome measures by occupying target sites with excess cold mass. However, the absolute injected mass levels were still quite low, and there was no evidence of a relationship between injected mass and 11C-PBR28 VT in any region (r2 < 0.025; P > 0.35). Therefore, mass effects likely did not influence these data. Second, given the low number of female smokers who participated in this study, it remains unknown whether male and female cigarette smokers have differences in TSPO availability. This question is particularly important in light of numerous sex differences in immune function (44) and is of high interest for future work. Because of this caveat, matching controls for subject sex and smoking status in future TSPO PET studies is still encouraged to account for these important factors (45). Third, the time of last nicotine exposure was not controlled in this study. Although no evidence of relationships between time of last cigarette and 11C-PBR28 VT were observed, this study was not sufficiently powered to systematically assess such possible effects. Finally, the effects of menthol cigarettes on TSPO availability were not investigated (46). These issues are important questions for future research.
CONCLUSION
Using rigorous quantitative methods we find no evidence of significant differences in TSPO availability (i.e., 11C-PBR28 VT) between tobacco smokers and nonsmokers. Future work is needed to elucidate fully specific immune consequences of tobacco smoking on the human brain.
DISCLOSURE
This work was funded by NIH grants K01 AA024788, R01 MH110674, and R01 DA038832 and by the VA National Center for PTSD. No other potential conflict of interest relevant to this article was reported.
KEY POINTS
QUESTION: Do TSPO levels differ between tobacco smokers and healthy nonsmokers?
PERTINENT FINDINGS: In comparing 11C-PBR28 volumes of distribution (estimated by kinetic modeling with metabolite-corrected arterial input function) in 20 tobacco smokers and 20 nonsmokers, we found no evidence of significantly different TSPO levels between the 2 groups.
IMPLICATIONS FOR PATIENT CARE: The findings suggest that possible immune consequences of tobacco smoking on the human brain are subtle. It is important to account for radiotracer concentrations in plasma for TSPO quantification with PET imaging.
Acknowledgments
We thank the staff at the Yale PET center for their expertise and support of radiochemistry and imaging. We are grateful to Jon Mikael Anderson and the Yale Translational Brain Imaging staff for their support of this work.
Footnotes
Published online Jan. 31, 2020.
- © 2020 by the Society of Nuclear Medicine and Molecular Imaging.
REFERENCES
- Received for publication October 4, 2019.
- Accepted for publication December 19, 2019.