Abstract
The rationale of this study was to investigate the performance of high-resolution CT (HRCT) versus 18F-FDG PET/CT for the diagnosis of pulmonary lymphangitic carcinomatosis (PLC). Methods: In this retrospective institution-approved study, 94 patients addressed for initial staging of lung cancer with suspicion of PLC were included. Using double-blind analysis, we assessed the presence of signs favoring PLC on HRCT (smooth or nodular septal lines, subpleural nodularity, peribronchovascular thickening, satellite nodules, lymph node enlargement, and pleural effusion). 18F-FDG PET/CT images were reviewed to qualitatively evaluate peritumoral uptake and to quantify tracer uptake in the tumoral and peritumoral areas. Histology performed on surgical specimens served as the gold standard for all patients. Results: Among 94 included patients, 73% (69/94) had histologically confirmed PLC. Peribronchovascular thickening, lymph node involvement, and increased peritumoral uptake were more often present in patients with PLC (P < 0.009). Metabolic variables, including tumor SUVmax, SUVmean, metabolic tumor volume, and total lesion glycolysis, as well as peritumoral SUVmax, SUVmean, and their respective ratios to background, were significantly higher in the PLC group than in the non-PLC group (P ≤ 0.0039). Sensitivity, specificity, and area under the receiver-operating-characteristic curve for peribronchovascular thickening (69%, 83%, and 0.76, respectively; 95% confidence interval [95%CI], 0.67–0.85) and increased peritumoral uptake (94%, 84%, and 0.89, respectively; 95%CI, 0.81–0.97) were similar (P = 0.054). For detecting PLC, sensitivity, specificity, and area under the receiver-operating-characteristic curve were significantly higher, at 97%, 92%, and 0.98, respectively (95%CI, 0.96–1.00), for peritumoral SUVmax and 94%, 88%, and 0.96, respectively (95%CI, 0.92–1.00), for peritumoral SUVmean (all P ≤ 0.025). Conclusion: Qualitative evaluation of 18F-FDG PET/CT and HRCT perform similarly for the diagnosis of PLC, with both being outperformed by 18F-FDG PET/CT quantitative parameters.
Pulmonary lymphangitic carcinomatosis (PLC) was first described by Troisier in 1873 and is morphologically defined by the presence of malignant cells within pulmonary vessels, in particular the lymphatics (1). Mainly originating from adenocarcinoma of the breast, stomach, lung, pancreas, and prostate, PLC may appear as a bilateral symmetric pattern in cases of hematogenous spread through the pulmonary arteries and subsequently into the perivascular interstitium and lymphatic vessels or as an asymmetric localized pattern with direct extension from primary lung tumor, hilar lymph nodes, or pleura (2). Even though a definitive diagnosis of PLC requires lung biopsy, high-resolution CT (HRCT) is considered an essential tool in the diagnostic process and is recommended before pathologic examination (3–5). The few studies that have investigated the role of 18F-FDG PET or PET/CT in the diagnosis of PLC suggest that PET/CT has an effective and reliable role as a noninvasive technique allowing identification of PLC with high specificity (6–8). Regarding the diagnostic performance of 18F-FDG PET/CT, data still remain spare compared with HRCT, and no study has formally evaluated the diagnostic value of quantitative PET/CT metrics compared with HRCT and PET/CT qualitative evaluations. We hence hypothesized that measurement of 18F-FDG uptake in the peritumoral lung may help to detect PLC in patients with primary lung cancer.
The aim of this study was to investigate the performance of HRCT versus 18F-FDG PET/CT for the diagnosis of PLC secondary to lung cancer, against the histologic gold standard, and to evaluate the added value of quantitative 18F-FDG PET/CT metrics.
MATERIALS AND METHODS
Study Design
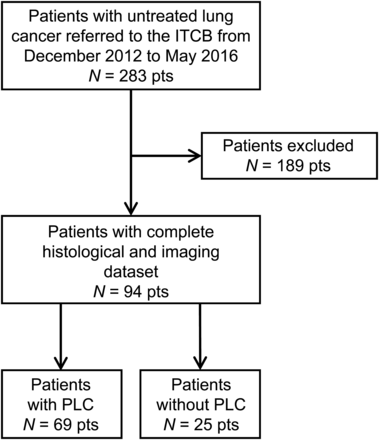
The study was conducted according to the 2015 Standards for Reporting of Diagnostic Accuracy Studies (9). All patients referred to our Institutional Thoracic Cancer Board from December 2012 to May 2016 after initial staging of untreated lung cancer were retrospectively reviewed. To be included, a patient had to have had both HRCT and 18F-FDG PET/CT within 10 wk of each other for initial staging and had to have had surgical resection (i.e., segmentectomy, lobectomy, or pneumectomy) without neoadjuvant chemotherapy. Patients were excluded if there had been more than 10 wk between HRCT and 18F-FDG PET/CT, if the 18F-FDG PET/CT had been performed at a different institution, or if neoadjuvant chemotherapy had been given (Fig. 1). PLC, as defined by the presence of secondary invasive cells within the vessels in the peritumoral area, was ascertained on pathology reports, which were used as our gold standard. For all included patients, the presence of imaging signs of PLC was evaluated on HRCT and 18F-FDG PET/CT based on a double-blind study and correlated with clinical pathology. The median delay between the 2 modalities was 3.5 wk (range, 0.1–10 wk). The local Ethics Research Committee of the State of Vaud approved the research protocol (CER-VD 2016-01295) and, because the study was retrospective, waived the need for informed consent.
Study flowchart. ITCB = Institutional Thoracic Cancer Board.
18F-FDG PET/CT Acquisition and Analysis
Patients underwent 18F-FDG PET/CT on a Discovery D690 TOF (GE Healthcare) 50–70 min after a 3.7 ± 0.5 MBq/kg intravenous injection of 18F-FDG. All patients fasted for at least 6 h and had blood glucose levels lower than 140 mg/dL before administration of 18F-FDG. A low-dose helical CT scan was first performed for anatomic correlation and attenuation correction (tube voltage, 120–140 kV; tube current, 80–200 mA or mA automodulation; pitch, 1.375; time per rotation, 0.8 s; slice thickness, 3.75 mm). Raw data were reconstructed using a blend of 40% adaptive statistical iterative reconstruction and 60% filtered backprojection. Whole-body emission images were then acquired using 7–9 overlapping bed positions of 2 min each (starting from the top of the skull and ending at the mid thigh). Images were reconstructed using ordered-subset expectation maximization (8 subsets, 2 iterations) with body weight–normalized SUV computation.
For each patient, the SUVmax (g/mL), SUVmean (g/mL), metabolic tumoral volume (cm3), and total lesion glycolysis (g⋅cm3/mL) of the primary lung tumor were measured using a standard 42% SUVmax threshold volume of interest embedding the whole tumor. In addition, perilesional tumoral activity was visually compared with contralateral normal lung (0, not increased; 1, increased) and quantified by measurement of SUVmax and SUVmean within a 3-cm peritumoral range using a 3 cm3 volume of interest on the most active region. Normal lung background uptake, as defined by SUVmean measured in a 3 cm3 volume of interest within the contralateral normal lung, was used to calculate peritumoral uptake ratios as follows: peritumoral SUVmax ratio = peritumoral SUVmax/contralateral normal-lung SUVmean. Peritumoral SUVmax and SUVmean were measured twice by 2 nuclear physicians with 4 and 10 y of experience, masked to the histologic results, to assess interobserver reproducibility.
HRCT Acquisition and Analysis
Thoracic HRCT was performed on multidetector CT scanners from multiple vendors. Because the study was retrospective, the dose parameters varied due to the different acquisition protocols used (tube voltage, 80–120 kV; tube intensity, 80–400 mA or mA automodulation). All raw data were reconstructed by filtered backprojection using a soft lung kernel with 1-mm slice thickness.
HRCT images of the 94 included patients were analyzed in consensus by 2 radiologists with 25 and 10 y of experience in thoracic imaging, with masking of the histologic results. The presence of smooth lines, nodular septal lines, subpleural nodularity, peribronchovascular thickening, satellite nodules, lymph node enlargement, pleural effusion, and enlarged pulmonary veins adjacent to the primary lung tumor was recorded at the patient level (0, absent; 1, present).
Histologic Analysis
All surgical specimens were retrospectively collected and prospectively analyzed by a pathologist with more than 15 y of experience in thoracic pathology. The pathologist was masked to the imaging results at the time of histologic analysis. Resected lung specimens were fixed in formalin for 24–48 h. Representative samples of the tumor were taken, embedded in paraffin, sectioned onto slides, and stained with hematoxylin and eosin. PLC was defined as the presence of secondary invasive cells within vessels in the peritumoral area. Tumor typing was performed according to the 2011 International Association for the Study of Lung Cancer/2015 World Health Organization classification (10).
Statistical Analysis
All statistical analyses were performed using STATA, version 15.1 (STATA Corp.). Sample size was calculated to test the equality of HRCT and 18F-FDG PET/CT. On the basis of a previous study by Prakash et al., we considered the accuracy of 18F-FDG PET/CT to be 93% and the difference between the 2 methods to be 10% (6). Using a 2-sided McNemar test at an α level of 0.05, we determined that a sample size of 93 patients would achieve 80% power. Continuous variables are presented as mean ± SD or as median and interquartile range (IQR). Categoric variables are presented as number or percentage. Histologic outcome was used as the gold standard for the diagnosis of PLC. All collected variables derived from the analysis of HRCT and 18F-FDG PET/CT were then compared between patients with and without PLC using the Kruskal–Wallis test for continuous variables and the Fisher exact test for categoric variables. The interobserver reproducibility of peritumoral SUVmax and SUVmean was assessed by the Pearson correlation coefficient (ρ) and the Lin concordance correlation coefficient (ρc = ρ × Cb, with Cb being a measurement of systematic bias) (11). The association between imaging variables and PLC was assessed using logistic regression analysis with computation of respective odds ratios (ORs) and 95% confidence intervals (95%CIs). The ORs of significant predictors were compared using the Hausman specification test. Receiver-operating-characteristic (ROC) curves were also analyzed, with computation of area under the ROC curve (ROC area), sensitivity, specificity, positive and negative likelihood ratios, and respective 95%CI for each variable. For continuous variables, optimal cutoffs allowing detection of patients with PLC were determined by the Liu method (12). ROC areas were compared for HRCT and 18F-FDG PET/CT variables that were significantly associated with PLC on univariate logistic regression analysis (i.e., peribronchovascular thickening, increased peritumoral uptake, and peritumoral SUVmax and SUVmean) using the nonparametric χ2 test of equality for ROC areas, as defined by DeLong et al. (13). For this comparison, we used multiple imaging parameters on univariate analysis, and the significance level was corrected by the Bonferroni method to account for multiple testing. P values of less than 0.003 were considered statistically significant.
RESULTS
Study Population
Overall, 94 patients (67 men and 27 women; median age, 68 y; range, 44–87 y) were retrospectively included (Table 1). All underwent surgical resection and histologic analysis of surgical specimens a mean of 5.9 wk (range, 0.6–22.8 mo) after the initial imaging evaluation. Of the 94 patients, 29 had stage 1 disease, 29 had stage 2 disease, 34 had stage 3 disease, and 2 had stage 4 disease according to the eighth edition of the TNM classification for lung cancer (14). Six patients underwent pneumonectomy, 81 patients lobectomy, and 7 patients segmentectomy. Fifty-five patients had adenocarcinoma, 34 patients squamous cell carcinoma, and 5 patients poorly differentiated non–small cell carcinoma. Histologic analysis additionally confirmed PLC in 69 of the 94 patients (73%).
Clinicopathologic Characteristics and Results of HRCT and 18F-FDG PET/CT Evaluation in Patients Without and With PLC
Qualitative HRCT and 18F-FDG PET/CT Analyses
All HRCT results are displayed in Table 1. There were no missing data for either imaging method. Only peribronchovascular thickening (Fig. 2) was significantly correlated with the presence of PLC (OR, 10.95; 95%CI, 3.33–36.0; P < 0.001; Table 2), showing an ROC area, sensitivity, specificity, and positive and negative likelihood ratios of 0.76 (95%CI, 0.67–0.85), 69%, 83%, 4.12, and 0.38, respectively. The presence of increased peritumoral uptake in comparison to lung background performed similarly to peribronchovascular thickening, with an ROC area, sensitivity, and specificity of 0.89 (95%CI, 0.81–0.97), 94%, and 84%, respectively (P = 0.054; Table 3).
Case of 66-y-old woman referred for initial staging of inferior lobe pulmonary adenocarcinoma (stage IIIA, arrow). (A) HRCT shows positive peribronchovascular thickening sign. (B) 18F-FDG PET/CT shows 18F-FDG uptake higher than background in corresponding region, with SUVmax of 2.5 g/mL and SUVmax-to-background ratio of 3.6.
Results of Association of HRCT Variables with PLC
Results of Association of 18F-FDG PET/CT Variables with PLC
Quantitative 18F-FDG PET/CT Analysis
The interobserver reproducibility of peritumoral SUVmax measurement (ρ = 0.922, ρc = 0.921, Cb = 1.0) and SUVmean measurement (ρ = 0.850, ρc = 0.850, Cb = 1.0) was excellent. Metabolic variables, including tumor total lesion glycolysis, peritumoral SUVmax, peritumoral SUVmean, and their respective ratios to background, were significantly higher in patients with PLC than in patients without PLC (P = 0.0006, 0.0001, 0.0001, 0.0001, and 0.0001, respectively; Table 1). Peritumoral SUVmax and SUVmean, but not tumoral quantitative parameters, were highly associated with PLC (Fig. 3; Table 3). Peritumoral SUVmax and SUVmean ORs were significantly higher than the ORs for qualitatively increased peritumoral uptake (P = 0.0022 and 0.0005, respectively) and than the ORs for peribronchovascular thickening (P = 0.0004 and P < 0.0001, respectively). Peritumoral SUVmax with a cutoff of 2.1 g/mL had a significantly higher sensitivity, specificity, and ROC area of 97%, 92%, and 0.98 (95%CI, 0.96–1.00), respectively, for detecting PLC than did either qualitatively increased peritumoral uptake (P = 0.0064) or peribronchovascular thickening (P < 0.0001; Fig. 4). Peritumoral SUVmean with a cutoff of 1.2 g/mL also had higher performance for detecting PLC than did increased peritumoral uptake (P = 0.025) or peribronchovascular thickening (P < 0.0001). The diagnostic performance of absolute peritumoral SUVmax, peritumoral SUVmean, peritumoral SUVmax ratio, and peritumoral SUVmean ratio was similar (P ≥ 0.10).
Box plot showing significant difference in SUVmax in peritumoral region on 18F-FDG PET/CT in patients with PLC (PLC+) and without PLC (PLC–).
ROC curves comparing performance of peribronchovascular thickening on HRCT, and performance of SUVmax in peritumoral region on 18F-FDG PET/CT, for diagnosis of PLC (P < 0.0001). Data in brackets are 95%CI.
DISCUSSION
In this study, we showed that 18F-FDG PET/CT and HRCT performed similarly in the diagnosis of PLC in patients addressed for initial staging of primary lung tumor when qualitatively evaluating, respectively, increased peritumoral uptake and peribronchovascular thickening. Other signs, such as smooth or nodular septal lines, subpleural nodularity, satellite nodules, and pleural effusion, were not significantly associated with PLC. Moreover, quantification of 18F-FDG uptake in the peritumoral area outperformed qualitative analysis of either modality.
The pathogenesis of pulmonary tumor embolism and lymphangitic carcinomatosis is poorly understood. It is thought that, in PLC, tumor cells gain access to the lung vascular system—and particularly to the lymphatic system—to induce local tumoral spread via the neovasculature or neolymphatics when the primary tumor is of the lung. When the primary tumor is nonpulmonary, the cells spread via retrograde flow since most of these malignancies involve the thoracic lymph nodes. Tumor cell invasion via the interstitial space or distended lymphatic vessels can result in thickened peribronchovascular bundles and septa (15–17). Tumor cells trapped within lymphatics result in local obstruction and fluid accumulation. Thus, peribronchovascular bundles and alveolar septal thickening may be due to local edema (1). Any malignant tumor has the potential to result in pulmonary tumor embolism, whether as PLC or as pulmonary tumor emboli, with a higher incidence in patients who have renal cell carcinoma, hepatocellular carcinoma, or adenocarcinoma of the breast, stomach, colon, or lung (18,19). This pattern of tumor spread is not common and occurs in less than 10% of metastatic cancers in the lung (2). The definitive diagnosis of PLC depends on the identification of tumor cells in the pulmonary lymphatics on histologic examination, which allows detection of obstruction and distension of pleural, peribronchial, perivascular, or subpleural lymphatics (17). PLC is typically an advanced-stage manifestation of malignancy and is associated with a poor prognosis (20–22).
Common findings associated with PLC on HRCT include thickening of interlobular septa and the peribronchovascular interstitium, subpleural nodules, thickening of the interlobar fissures, pleural effusion, pleural carcinomatosis, and hilar and mediastinal nodal enlargement, with relatively little destruction of overall lung architecture (4). However, smooth or thickened interlobular septa on HRCT, in particular with a nodular appearance, are not specific for PLC because these findings are also encountered in other interstitial disorders, such as sarcoidosis (23). Hilar adenopathy and effusions were occasionally present in reported series of PLC (24).
In a retrospective study on 21 patients, when correlated with pathology results, certain characteristic findings on CT scans were evident: uneven thickening of peribronchovascular bundles, thickening of isolated interstitial lines, and the presence of polygonal lines. The pathologic basis for these characteristic CT findings was considered by the authors as related to tumor thrombi in lymphatic vessels rather than to edema and fibrosis, at least in the early stages of disease (25). Another study reported that thickening of peribronchovascular bundles and interlobular septa is the single most important chest CT finding of PLC (26). Our study hereby confirms that peribronchovascular thickening and lymph node enlargement should be considered signs of PLC mainly at initial staging; it is the first report of diagnostic performance of HRCT in this setting to our knowledge.
Regarding 18F-FDG PET/CT, to our knowledge we have described the largest series of patients who had histologically proven PLC and underwent imaging with a state-of-the art 18F-FDG PET/CT scanner. To the best of our knowledge, only 3 studies have reported the role of 18F-FDG PET/CT in the diagnosis of PLC. In a retrospective study of 35 patients, Prakash et al. found a high specificity of 100% for 18F-FDG PET/CT in the detection of PLC, with a sensitivity of 86% (6). In addition, that study reported a statistically significant increased 18F-FDG uptake in the PLC areas in contrast to normal lung parenchyma and blood-pool activity, in concordance with our results. In a group of 7 patients with PLC, Digumarthy et al. reported that the intensity of 18F-FDG uptake was significantly greater in diseased lung than in corresponding normal contralateral lung or in the lungs of healthy controls, with the SUV ratio for diseased to normal tissue being significantly increased (8). In a series of 5 PLC-positive cases, Acikgoz et al. described the 18F-FDG PET/CT pattern as varying from a diffuse, lobar, or segmental 18F-FDG uptake in the lungs in extensive PLC to a hazy area of 18F-FDG uptake or linear uptake extending from the tumor to the lymph nodes in limited PLC (7). The route of lymphangitic spread in these cases was considered to be either through seeding of tumor cells to the peribronchovascular lymphatics or through direct invasion of lymphatics by the lung tumor. Although we confirmed that increased peritumoral uptake may be useful in the detection of PLC, its performance was not superior to that of peribronchovascular thickening on HRCT. Moreover, we demonstrated that adding peritumoral SUV and ratio to lung background measurement significantly improved the performance of 18F-FDG PET/CT for the diagnosis of PLC. Quantitative 18F-FDG PET/CT of peritumoral areas should hence be part of the initial imaging workup of patients with primary lung cancer. However, CT images should always be checked before peritumoral SUV is measured, to avoid misinterpreting peritumoral infiltrates for another etiology, such as infection (27). In hybrid imaging, acquisition of HRCT images during a single contrast-enhanced 18F-FDG PET/HRCT examination should be the next step to optimize patients’ evaluation and minimize costs and needs to be investigated further.
We have to address some limitations of our study. First, because the study was retrospective, HRCT images were acquired on CT scanners from multiple vendors and with variable acquisition parameters but similar reconstruction parameters. Although image quality may differ, such variations reflect the daily practice of our Institutional Thoracic Cancer Board. Second, HRCT images were assessed by experts in thoracic imaging. Although qualitative evaluation of HRCT and 18F-FDG PET/CT demonstrated similar performance, the learning curve to detect subtle peribronchovascular thickening on HRCT may be an issue in reproducing this result in daily practice, especially in situations such as COPD patients with bronchial wall thickening. Third, only patients who underwent surgical resection were included. Although this criterion allowed histologic confirmation of PLC, reported cutoffs for quantitative metrics may not be fully transposable to inoperable patients or to the use of different PET scanners or reconstruction algorithms. However, use of peritumoral SUV ratios may overwhelm this limitation, with similar diagnostic confidence. Finally, whereas the use of 18F-FDG PET/CT quantitative parameters improved the diagnostic performance of 18F-FDG PET/CT for PLC, the prognostic value of these metrics at initial staging remains unknown. This area of investigation was outside the scope of the present study and should be further assessed to fully evaluate the usefulness of these measurements.
CONCLUSION
Our study showed that peribronchovascular thickening and increased peritumoral tracer uptake on, respectively, HRCT and 18F-FDG PET/CT performed similarly for the diagnosis of PLC in patients with lung cancer at initial staging. Peritumoral 18F-FDG uptake quantification, however, outperformed qualitative evaluation. Combining the resultant morphologic and metabolic criteria may thus help to establish a powerful tool for the diagnosis of PLC.
DISCLOSURE
No potential conflict of interest relevant to this article was reported.
KEY POINTS
QUESTION: How do the performance of HRCT and 18F-FDG PET/CT compare for the diagnosis of PLC?
PERTINENT FINDINGS: Peribronchovascular thickening and increased peritumoral tracer uptake on, respectively, HRCT and 18F-FDG PET/CT perform similarly for the diagnosis of PLC in patients with lung cancer at initial staging. Peritumoral 18F-FDG uptake quantification, however, outperforms qualitative evaluation on either modality.
IMPLICATIONS FOR PATIENT CARE: Combining morphologic and metabolic parameters may help establish a powerful tool for the diagnosis of PLC.
Footnotes
Published online Jun. 21, 2019.
- © 2020 by the Society of Nuclear Medicine and Molecular Imaging.
REFERENCES
- Received for publication April 9, 2019.
- Accepted for publication June 5, 2019.