Abstract
645
Purpose: The use of 18F- fludeoxyglucose (FDG) PET to monitor treatment with immune checkpoint inhibitors has been questioned because new inflammatory lesions may occur as part of the immune response and infiltration of the tumor by immune cells may cause a transient increase in metabolic activity or cause new inflammatory lesions to appear on FDG PET. However, there are only few systematic studies on this subject. Thus, the purpose of this retrospective study was to investigate the relationship between changes in tumor 18F-FDG uptake and survival in patients with advanced melanoma undergoing immunotherapy with PD-1 antibodies. Materials and Methods: One hundred sixty-six PET/CT scans in 83 patients (patients, median age 67 years; range 17 - 93 years) who were treated with PD-1 blockade (nivolumab plus ipilimumab (n=28) and pembrolizumab (n=55)) and underwent 18F-FDG PET/CT before and after treatment were included in this analysis. All patients had advanced melanoma and underwent baseline imaging within 2 months before initiation of PD1 blockade, and follow-up 18F-FDG PET/CT no more than 4months after the start of PD1 blockade. Treatment response on FDG PET was assessed according to PERCIST based approach: by measuring % change in SULpeak of the 5 lesions with the highest SUL on the baseline image (maximum of two per organ) and on the follow-up scan (not necessarily the same lesion). Changes in tumor FDG uptake were classified according to PERCIST criteria as complete and partial metabolic response (CMR, PMR), stable metabolic disease (SMD) and progressive metabolic disease (PMD). PMD was defined in two different ways. By the first definition any new FDG avid lesion suspicious for malignancy was considered to represent PMD (conventional PERCIST: PERCIST). In the second approach, the appearance of new lesions alone was not considered as PMR (immunotherapy-modified PERCIST: imPERCIST). PET/CT responses were correlated with overall survival (OS) using Kaplan-Meier curves and log-rank tests.
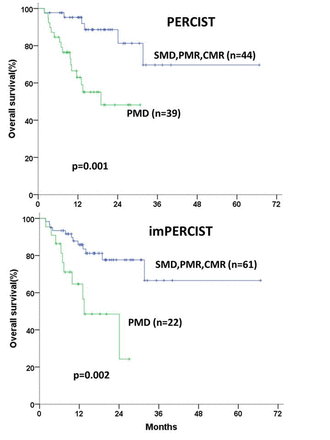
Results: Median OS of all patients was not reached. By PERCIST thirteen patients showed CMR, 25 patients PMR, 6 patients SMD, and 39 patients PMD. When new lesions were not considered to represent PMD, the number of patients with PMD by imPERCIST decreased by 18 (shifted to 12 PMR, 6 SMD). Conversely one patient with SMD by PERCIST shifted to PMD by imPERCIST. Responses by imPERCIST and PERCIST were identical in 62 of 83 patients (κ = 0.65, table 1). Two approaches for response assessment on FDG PET showed similar correlations with OS (figure 1). However, the median OS of PMD groups by imPERCIST was shorter than that by PERCIST (13.47 months vs 18.96 months)
Conclusions: In this retrospective study of patients with advanced melanoma treated with PD-1 antibodies, tumor response by standard PERCIST was significantly correlated with OS. Modifications of the PERCIST criteria to account for inflammatory lesions did not substantially improve the prognostic value of tumor response on FDG PET. While these results are promising for the use of FDG PET for monitoring immunotherapy, further studies are warranted to understand the impact of the type of immunotherapy and the timing of the FDG PET scans on response assessment.
Table1