Abstract
1252
Background: Although classical Hodgkin lymphoma (cHL) is highly curable, 20%-30% of patients will not be cured with conventional treatments. The programmed death‐1 (PD‐1) receptor checkpoint inhibitors represent an important therapeutic advance in the treatment of relapsed or refractory cHL. Tislelizumab or Camrelizumab (BeiGene and Henrui Pharma, Beijing and Lianyungang, China), two highly selective, fully humanized, monoclonal antibodies, block the interaction between PD-1 and its ligands. We aimed to assess the tumor response and outcome profile of Tislelizumab and Camrelizumab in Chinese patients with relapsed or refractory classical Hodgkin lymphoma.
Methods: In this ongoing, single-arm, phase 2 studies, we recruited patients with histopathologically diagnosed classical Hodgkin lymphoma that was relapsed or refractory after two or more lines of therapy. Patients were given intravenous Tislelizumab or Camrelizumab (200 mg, once every 2 weeks) until progression, death, unacceptable toxicity, or withdrawal of consent. For Tislelizumab, 18F-FDG PET/CT scans were acquired at baseline, at weeks 13, 25, and every 30 weeks from weeks 56. For Camrelizumab, PET/CT scans were acquired at baseline, at weeks 17, 25 and/or 48. We recorded the best overall response according to the International Harmonization Project Cheson 2014 criteria and LYmphoma Response to Immunomodulatory therapy Criteria (LYRIC) (2016 revised criteria). Patients achieving an objective response at any time during the anti-PD1 treatment were classified as responders. Safety was assessed in all treated patients.
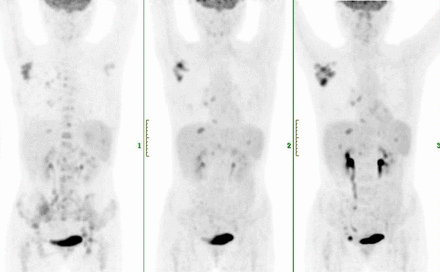
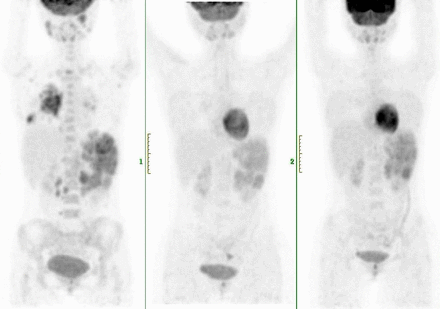
Results: Between May 8, 2017, and Dec 1, 2018, 38 relapsed or refractory classic HL patients were included. The median age was 32 y (age range, 18-64 y). The median previous lines of therapy were 3 (range, 2-6). The mean follow-up was 14.6 mo. Thirty-two of 38 patients (84%) achieved an objective response, and 50% of those achieved a complete response. The treatment-related adverse events were mild, and 5 patients (13%) had grade 2 or 3 treatment-related adverse events, the most common being pneumonitis (three [8%] patients). One (3%) patient had myocarditis, and one patient had renal injury. No patient died during the study.18F-FDG PET/CT detected all responders at 4 mo. Decrease in tumor metabolism and volume (SUVmean, metabolic tumor volume) were observed in responders. Six of 38 patients (15%) displayed new imaging patterns related to anti-PD1; we observed 2 transient progressions consistent with indeterminate response according to the LYRIC (2016) (IR3 at 13 weeks).
Conclusions: Tislelizumab or Camrelizumab could be a new treatment option for patients with relapsed or refractory classical Hodgkin lymphoma in China. Three-month 18F-FDG PET/CT scans detected HL patients responding to anti-PD1. New patterns were encountered in 15% of patients, emphasizing the need for further evaluation in larger series and close collaboration between imaging and oncology specialists on a per-patient basis.