Abstract
246
Objectives: Surgical treatment of low rectal cancer includes two standard
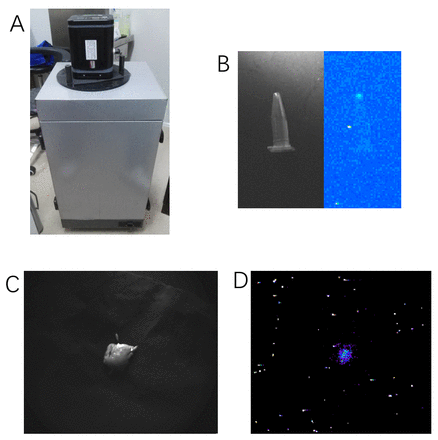
Methods: Miles operation and Dixon operation. For Miles operation, it usually seriously reduces the patient's quality of life after surgery, though it ensures the radical resection. Whereas for Dixon operation, it can improve the patient quality of life, however it probably has the risk of residual tumor which may lead to the recurrence. It is recommended in the NCCN guidelines that intraoperative frozen pathology is used for ensuring that there is no residual tumor. However, intraoperative frozen pathology usually takes long time and the results are not always accurate because of the limitation of the techniques. Therefore, a novel intraoperative imaging method is needed for helping surgeon to determine whether there is residual tumor. Cerenkov luminescence imaging (CLI) has attracted more and more attentions in recent years because it has many advantages: the wide variety of clinically available imaging probes, high superficial resolution, high imaging throughput capability, and low cost. In this study, the construction and evaluation of a novel intraoperative CLI system was reported for guiding the accurate resection of low rectal cancer using Dixon surgery in swine models. Methods Firstly, a novel intraoperative CLI system was constructed. It was mainly consisted of a highly sensitive camera (iXon3 888,ANDOR,UK) and a lens (Xenon 0.95/17-0010, Schneider, Germany), which were used for acquiring Cerenkov luminescence during the operation. Secondly, many imaging parameters of the system were tested, including the work distance (WD), field of view (FOV), sensibility, and resolution. Thirdly, the phantom study using an Eppendorf (EP) tube contained 2-deoxy-2-[18F]-fluoro-D-glucose (18F-FDG) (1.0 μCi, 0.004 ml) was conducted. Both the CL image and the photograph were acquired (exposure time: 5 mins for CLI; 0.5 s for photograph). Lastly, 18F-FDG (6 μCi, 2.4 ml) was injected into the intestinal mucosa of the normal swine (n=2) using an endoscope to simulate the radioactivity uptake in low rectal cancer. Then the simulated lesion was removed by an experienced colon cancer surgeon and placed in the intraoperative CLI system for acquiring CL signal (exposure time: 5 mins for CLI; 1 s for photograph). CL images were processed by use of median filtering for gamma ray rejection and then fused with the photograph image. The Student t test (Prism v6.0, GraphPad, La Jolla, CA) was used to determine statistical significance. Results The intraoperative CLI system was developed [Figure 1A]. The optimized imaging parameters of the system were included in Table 1. The photograph and the CL image of 250 µCi/ml 18F-FDG were clearly visualized using the system [Figure 1B]. Figure 1C represents the photograph and CL image of the resected simulated low rectal cancer tissue. Conclusions An intraoperative CLI system with high resolution and sensitivity was successfully developed for image-guided resection of low rectal cancer on swine models. The intraoperative CLI system exhibits high potential for image-guided resection of low rectal cancer in clinic.
Parameters of the intraoperative CLI system