Abstract
601
Objectives: Pancreatic cancer and glioma are among the most lethal solid tumors with locally aggressive nature and early metastasis, resulting in grim prognosis even after surgery which is the only curative option for both. Epidermal growth factor receptor (EGFR) is overexpressed in 40-60% of pancreatic cancers and most of glioma and can serve as a potential target for the treatment of both tumour. Here we designed and developed a smart drug delivery system for the theranostics of both pancreatic cancer and glioma. Specifically, six arginine linked with anti-EGFR affibody ZEGFR:1907 (A6) was applied to modify the ultra-small 11-mercaptoundecanoic acid gold nanoclusters (MUA-Au NCs) (Figure A). The low-toxic fluorescent MUA-Au NCs render excellent ability for imaging specific targets, also with excellent tumour treatment properties when anti-cancer drugs are loaded into the nanoclusters. Methotrexate (MTX) as a commonly used anti-cancer drug and a folate receptor targeting agent is loaded into A6MUA-Au NCs, forming A6MUA-Au NCs-MTX (AMAM). The resulting dual targeting nanodrug carrier can be controlled to release MTX in tumor. With low cost and simplicity of carrier fabrication, precision of tumour targeting and adequate drug loading capacity, as well as controlled drug release at tumour sites, AMAM is expected to be a promising agent for targeted imaging and treatment of pancreatic cancer and glioma.
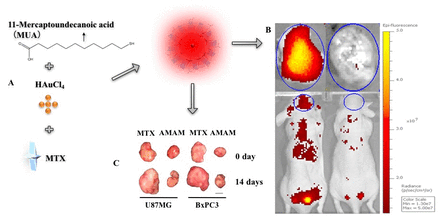
Methods: Au NCs was synthesized using chloroauric acid and MUA via one-pot synthesis. A6 were then used to functionalize MUA-Au NCs followed by loading MTX into the nanoclusters for fabricating the multifunctional smart drug delivery system AMAM (Figure A). U87MG and BxPC3 cells were injected into groups of mice (n=16 each group) for establishing orthotropic and xenografts tumour models. Fluorescence images were then acquired after incubation of AMAM with cultured U87MG and BxPC3 cells separately from 1 h to 12 h for in vitro imaging. In vivo fluorescent imaging was performed from 1 h to 72 h after tail vein injection of AMAM into tumour planted mice (n=4 per group). The drug released properties, cytotoxicity of AMAM in vitro and tumour treatment effects in vivo were investigated in mice bearing U87MG and BxPC3 (n=4 per group). All data were analysed by SPSS.
Results: MUA-Au NCs were fabricated as 2 nm average size and 0 zeta potential, and after functionalized by A6, the size increased to about 20 nm and zeta potential decreased to about -30 mV, suggesting the formation of a more stable nanoclusters. The maximal absorption and fluorescence emission wavelengths of A6MUA-Au NCs were 535 nm and 640 nm, respectively. MTX was loaded into the A6MUA-Au NCs with a decent loading efficiency (35.05%). EGFR overexpressed U87MG and BxPC3 cells efficiently took up AMAM, and the nanomaterial successfully released MTX into cells, resulting in 59.61% and 84.63% higher anti-U87MG and BxPC3 cells activity than MTX alone at 24 h incubation, respectively. Moreover, fluorescence image analysis also revealed that AMAM displayed high accumulation in U87MG and PxPC3 xenografts, with tumor-to-background contrast ratio significantly increased over two folds compared to the use of non-targeted MUA-Au NCs-MTX (p<0.05). Pre-injection of ZEGFR:1907 before the administration of AMAM significantly reduced the tumor imaging signal, showing EGFR targeting specificity of AMAM (Figure B). Lastly, treatment study showed that tumor size decreased by AMAM for 14 days around 35% and 50% compared to MTX used only on U87MG and BxPC3 xenografts tumor models, respectively (Figure C).
Conclusion: AMAM can be efficiently delivered to EGFR overexpressed tumor cells, subsequently releases MTX efficiently for cancer treatment both in vitro and in vivo. It is anticipated that the dual targeted nanomaterial can serve as a generalized strategy for multi-modality imaging and tumor-targeted drug delivery. Research Support This research is supported by the Office of Science (BER) and US Department of Energy (DE-SC0008397).