Abstract
Cardiac CT angiography has become an important tool for the diagnosis and treatment of congestive heart failure. Differentiation of ischemic from nonischemic cardiomyopathy; evaluation of myocardial perfusion; characterization of hypertrophic cardiomyopathy, left ventricular noncompaction, and arrhythmogenic right ventricular dysplasia; and delineation of congenital heart defects and valvular abnormalities are the primary diagnostic applications. Therapeutic use includes visualization of the coronary venous anatomy for optimal implementation of cardiac resynchronization therapy and evaluation of left ventricular assist devices and transplant vasculopathy.
Heart failure is a complex clinical syndrome that results from any structural or functional impairment of ventricular filling or ejection of blood (1). It affects approximately 5.1 million people in the United States, with greater than 650,000 cases diagnosed annually, and those numbers are expected to rise in the future (2). Heart failure is a progressive disease that leads to significant morbidity and mortality and accounts for more than 1 million hospitalizations annually, with a 5-y mortality of approximately 50% (3). Multidetector cardiac CT angiography (CTA) is an ever-advancing technology, having evolved from the early 4-slice acquisition systems to the modern multislice cardiac CT. With up to 320 simultaneous slices and faster gantry rotational systems, cardiac CT allows for improved temporal and spatial resolution. Entire examinations can be completed in less than 5 s, and the volume of contrast material required for comprehensive examinations continues to decrease. This review summarizes the many potential applications of CTA for patients with heart failure.
DIAGNOSIS OF HEART FAILURE
Reduced Ejection Fraction (EF)
The identification of reduced left ventricular (LV) function is at the core of the diagnosis of heart failure with reduced EF. Reduced LV function can be detected by multiple modalities, including transthoracic echocardiography, the modality most commonly used for diagnosis; transesophageal echocardiography; cardiac MR imaging; left ventriculography during cardiac catheterization; radionuclide ventriculography; and SPECT.
CTA can also be used to diagnose reduced LV function. With retrospective imaging techniques, continuous multidetector image acquisition with electrocardiogram gating allows for the reconstruction of images at multiple instants in the cardiac cycle. With short-axis reconstruction at every 5% or 10% of the R-R interval, both LV and right ventricular (RV) volumes can be calculated, allowing for determination of the EFs of both ventricles (Fig. 1). EF calculation by CTA correlates well with echocardiographic assessment of LV function (4). Multiple studies have also shown a strong correlation between CTA and cardiac MR imaging for EF calculation (5–7). However, CTA has a more limited temporal resolution than cardiac MR imaging, resulting in slight overestimations of end-systolic volume and EF, especially in patients with a reduced EF (7). Nonetheless, according to American College of Cardiology Foundation/American Heart Association guidelines for the diagnosis and management of heart failure, CTA may be useful for assessing cardiac function and wall motion (1).
Nonischemic cardiomyopathy in 65-y-old man who had new-onset dyspnea on exertion and atypical chest pain. Cardiac CTA revealed no significant obstructive disease and ejection fraction of 25%. Cardiac catheterization was not performed. (Top) End-diastolic (ED) and end-systolic (ES) frames. (Middle) LV volume curve. (Bottom) Derived CAT measurements. HR = heart rate.
Regional Wall Motion Abnormalities
Aside from measuring overall contractility, CTA is also useful for the diagnosis of regional wall motion abnormalities. With a cine-loop format to display images, regional wall motion can be evaluated. Several studies have confirmed the usefulness of CTA for evaluating LV regional wall function (4,8–10). CTA has a high specificity for detecting wall motion abnormalities, and it appears to have the highest accuracy for detecting wall motion abnormalities in the left anterior descending and left circumflex arteries (11). Its accuracy for the identification of regional wall motion abnormalities approaches the accuracy of cardiac MR imaging (12).
The ability of CTA to measure ventricular volumes and EF is not limited to the left ventricle. CTA can be useful for evaluation of the right ventricle, which can be technically difficult to evaluate by other imaging modalities, especially echocardiography. RV volumes and EF calculations have been shown to correlate well with those obtained from cardiac MR imaging, with a correlation coefficient of greater than 0.96 (13). Therefore, for patients with limited echocardiographic windows or for patients who cannot undergo cardiac MR imaging because of device implantation, CTA is currently recommended for the evaluation of RV morphology and function (14).
Heart Failure with Preserved EF
Heart failure encompasses more than just reduced LV function. It has been estimated that approximately 50% of patients with clinical heart failure have normal systolic function (1). This disease entity, termed heart failure with preserved EF, is the result of abnormalities in myocardial diastolic properties. Multiple echocardiographic indices, including mitral valve flow velocities and tissue Doppler velocities, are used for the diagnosis of heart failure with preserved EF. However, it may be feasible to use CTA for measurement of the diastolic properties of the heart. In an elegant study, Boogers et al. (15) used mathematic analyses to calculate transmitral valve flow velocities and mitral valve ring motion velocities at the septum. These measurements showed a good correlation with echocardiographically derived measures of diastolic function, with an overall accuracy of 79%. Although not currently used for this disease process, CTA may play a role in the diagnosis of heart failure with preserved EF in the future, especially for patients already undergoing CTA for coronary artery evaluation.
ETIOLOGY OF HEART FAILURE
Once the diagnosis of heart failure has been established, it is important to establish the etiology of the disease. There are many possible underlying causes for heart failure, both cardiac and noncardiac. Identification of the condition responsible may be important because some conditions that lead to LV dysfunction are potentially treatable or reversible.
Ischemic Versus Nonischemic Cardiomyopathy
One of the most important initial diagnostic steps in the workup of heart failure is the distinction between ischemic cardiomyopathy and nonischemic cardiomyopathy. The belief that coronary artery disease (CAD) is the underlying cause in approximately two-thirds of patients with heart failure (16) has important therapeutic and prognostic implications, as revascularization can favorably affect LV function in some patients with impaired yet viable myocardium (17).
It is difficult to clinically distinguish between ischemic cardiomyopathy and nonischemic cardiomyopathy without further diagnostic workup. Patients with nonischemic cardiomyopathy may have symptoms of chest pain or electrocardiogram changes consistent with CAD. In contrast, patients with ischemic cardiomyopathy may have heart failure and no symptoms of chest pain. In addition, specific echocardiographic findings may not always be reliable in helping to establish a diagnosis. Segmental wall motion abnormalities are common in dilated cardiomyopathy, even in the absence of obstructive CAD (Fig. 1) (18).
Invasive coronary angiography (ICA) is considered the gold standard for the diagnosis of ischemic cardiomyopathy. In patients with newly diagnosed heart failure and anginal symptoms, coronary angiography is recommended (1). However, ICA is an invasive study that carries a risk for complications, most importantly, vascular access site complications, with bleeding rates ranging from 0.05% to 2.3%, as well as death, myocardial infarction, and stroke, with rates approaching 3% (19). Therefore, noninvasive modalities are a reasonable initial diagnostic step (1).
Assessment of coronary artery calcium (CAC), without the need for intravenous contrast material administration, can provide important diagnostic data. Multiple studies have shown the value of CAC scores in excluding CAD as the etiology for heart failure (20–22). In patients with a diagnosis of heart failure, an Agatston score of 0 has been shown to have 100% specificity in excluding “high-risk CAD,” defined as left main coronary artery stenosis or stenosis in at least 2 major epicardial coronary arteries (20,22). Although the clinical utility of CAC scores in patients with heart failure has not been well described, assessment of CAC should be considered as an initial study to exclude an ischemic etiology for heart failure.
Beyond providing an assessment of CAC, CTA can provide a direct noninvasive evaluation of the coronary arteries with intravenous contrast material. Compared with invasive angiography, CTA had high qualitative and quantitative accuracy in the general population (23). CTA has also been shown to be feasible, safe, and accurate for detecting CAD with high sensitivity and specificity in patients with dilated cardiomyopathy (24). A metaanalysis by Bhatti et al. (25) demonstrated that CTA is a highly accurate diagnostic modality for excluding an ischemic etiology in patients with cardiomyopathy of undetermined cause, with a sensitivity of 98% and a specificity of 97%. In fact, CTA has been accepted as an alternative to ICA for evaluating coronary arteries in patients with new-onset heart failure to assess etiology (25). In an ongoing randomized clinical trial, IMAGE HF Project 1-C, CTA and ICA are being compared for patients with heart failure requiring coronary anatomic definition, endpoints of diagnostic accuracy, clinical outcomes, and resource use (26).
The use of CTA for the assessment of coronary arteries is not limited to patients with normal sinus rhythm. Atrial fibrillation is a common comorbid condition in patients with heart failure, with a prevalence approaching 50% in patients with more advanced heart failure (27). Patients with atrial fibrillation have been excluded from most CTA studies because the test requires a low heart rate, and the beat-to-beat variations in atrial fibrillation can lead to poor image quality and make image reconstruction difficult. Nonetheless, a metaanalysis by Vorre and Abdulla (28) showed that CTA has high diagnostic accuracy in patients with atrial fibrillation. However, patients with atrial fibrillation did require a higher effective radiation dose than patients with normal sinus rhythm.
CTA can also provide more than just an assessment of the coronary arteries in patients with ischemic cardiomyopathy. With first-pass injection of contrast material, CTA imaging has the ability to determine resting myocardial perfusion defects. Compared with myocardial perfusion imaging, CTA accurately detected perfusion defects (29). Compared with ICA, CTA had a low positive predictive value, likely the result of image artifacts; however, it had a notable ability to exclude flow-limiting lesions, with a negative predictive value of greater than 85% (30).
Beyond the differentiation between ischemic cardiomyopathy and nonischemic cardiomyopathy, other cardiomyopathy subtypes have distinctive changes that can be detected by CTA. In infiltrative cardiomyopathies, such as amyloidosis or sarcoidosis, subtly heterogeneous attenuation of the myocardium can be seen on CTA. In hypertrophic cardiomyopathy, CTA can show increased wall thickness and can delineate the exact location and extent of myocardial involvement. LV noncompaction can also be identified by CTA, with prominent trabeculations and areas of noncompaction (Fig. 2). In addition, in arrhythmogenic RV dysplasia, CTA can demonstrate fat in the RV wall and abnormal RV wall motion (Fig. 3) (31).
LV noncompaction. (Left) Axial image of heart shows deep intertrabecular recesses (arrows) at apex and lateral wall of left ventricle. (Right) Short-axis volume–rendered image of heart shows areas of low Hounsfield units within LV wall (arrows), which are likely to be fibroelastic infiltrations of noncompaction. LA = left atrium; RV = right ventricle.
Arrhythmogenic RV dysplasia. Arrows highlight areas of fat in dilated right ventricle (RV). LV = left ventricle.
CTA can also be useful for patients with congenital heart disease. More patients with congenital heart defects are living into adulthood and, not uncommonly, can develop heart failure as a consequence of their underlying cardiac abnormalities. CTA can provide a high-quality assessment of congenital anatomy, prior surgical repair, and progression of underlying congenital conditions as well as structural and anatomic details of coronary artery anomalies (32,33).
Valvular Heart Disease
Valvular heart disease is a common cause of heart failure. A full description of the use of CTA for the diagnosis and management of valvular heart disease is beyond the scope of this review, but a brief discussion of the use of CTA for left-side valvular lesions, the most common cause of valve-related heart failure, is warranted.
CTA can be useful for the assessment of aortic valve lesions. In aortic regurgitation, the aortic regurgitant orifice area can be calculated by planimetry with CTA, and quantification of the regurgitant orifice area has been shown to correlate well with echocardiography in determining the severity of aortic regurgitation (34). The severity of aortic stenosis can also be evaluated effectively by use of CTA for planimetry of the aortic valve area during the systolic phase. Compared with transesophageal echocardiography, CTA provided an accurate measurement of the aortic valve area (35). Beyond diagnosis, CTA can provide critical treatment-related information for aortic valve disease. A comprehensive CTA examination is part of the standard preprocedural protocol at many institutions when transcatheter aortic valve replacement is being considered.
Mitral valve lesions can also be evaluated by CTA. In mitral stenosis, CTA shows thickened leaflets, and planimetry can be used to measure the mitral valve area. Studies have shown that although the mitral valve area measured by CTA planimetry is systematically larger than that calculated by echocardiography, CTA may allow reliable discrimination of mitral valve stenosis severity grades (36). In mitral regurgitation, CTA can show a lack of apposition of the mitral valve cusps at the end of systole, and it allows for direct planimetry of the regurgitant orifice area. CTA also allows for the detection of structural abnormalities of the mitral valve, such as prolapse, flail leaflet, annular calcification, and leaflet thickening. Moreover, in functional mitral regurgitation, CTA can provide anatomic and geometric details of the entire mitral valve apparatus, yielding both diagnostic and therapeutic information.
TREATMENT OF HEART FAILURE
Beyond the etiology of heart failure, CTA is extremely valuable for various heart failure treatment modalities, including cardiac resynchronization therapy (CRT), left ventricular assist devices (LVADs), and heart transplants.
CRT
CRT via the implantation of a biventricular pacemaker is a therapeutic option in symptomatic patients with LV dysfunction and a wide QRS complex. This therapy has been shown to improve symptoms and ventricular size and function while decreasing hospitalizations and mortality (37–39). However, a significant percentage of patients receiving CRT therapy do not show any symptomatic benefit and are termed “CRT nonresponders.” Among the critical issues in determining the success of CRT therapy are LV dyssynchrony and cardiac venous anatomy.
CRT therapy involves the recoordination of regional wall contraction, and the presence of LV dyssynchrony at baseline appears to be an important predictor of the response to CRT (40). Echocardiography is the conventional method for the evaluation of LV mechanical dyssynchrony. However, CTA is also able to provide an assessment of mechanical dyssynchrony and was shown to be accurate in a comparison with echocardiography (41). CTA has been proven to be helpful in guiding CRT therapy on this basis (42).
The implantation of a CRT device involves the insertion of an LV pacing lead through the coronary sinus, and the main factor determining the success of LV lead implantation is cardiac venous anatomy (43). A careful assessment of venous anatomy ensures that the latest area of ventricular activation can be reached with the pacemaker lead. The technique most commonly used to evaluate the coronary venous system is retrograde venography by direct manual contrast material injection, but several studies have shown the feasibility of CTA for the noninvasive assessment of cardiac venous anatomy (Fig. 4) (42,44,45). In particular, in patients with abnormal venous anatomy, CTA can provide valuable information on the course of the coronary sinus and its tributaries. CTA can also identify patients in whom surgical epicardial lead placement may be more appropriate from the outset. Moreover, as demonstrated in an elegant study by Wong et al. (46), CTA can also be used for postimplant assessment of lead placement, especially in relation to areas of myocardial scarring, which has been associated with a nonresponse to CRT therapy.
Low origin of lateral vein close to coronary sinus (CS) ostium. (Left) Volume-rendered image. (Right) Axial image.
LVAD Assessment
Because of the growing burdens of heart failure and donor organ shortage and a rise in the number of elderly patients ineligible for heart transplants, LVADs have emerged as an increasingly viable therapy for advanced heart failure (47). They provide a significant mortality benefit over conventional medical therapy; more than 2,000 LVADs were implanted in the year 2013 alone (48). However, with the significant increase in the use of LVADs comes the potential for an increased incidence of associated complications, and detection and evaluation of these complications have important therapeutic implications.
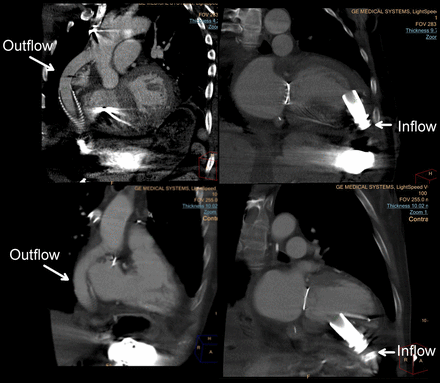
Although other imaging modalities are unable to provide a comprehensive assessment of all LVAD components, CTA provides noninvasive high-resolution imaging of LVADs in their entirety (Fig. 5). The volume of coverage provided by CTA enables direct visualization of inflow and outflow cannulas in their entirety and allows for the evaluation of LVAD placement as well as the recognition of cannula thrombus or deformation. Although some degree of imaging artifacts can be generated by an LVAD, CTA can be used to diagnose LVAD inflow obstruction; multiple studies have proven CTA to be feasible and accurate for detecting inflow and outflow cannula thrombosis and malposition (49,50).
LVADs. (Top) Outflow and inflow with typical apical inflow location. (Bottom) Outflow and inflow with atypical interior inflow location.
Another application of CTA in patients with LVADs involves assessment of the right ventricle. After LVAD implantation, right heart failure confers significant morbidity and mortality (51). An accurate echocardiographic assessment of RV size and function after LVAD implantation can be technically difficult because of postoperative changes and artifacts from the LVAD itself. CTA, without the limitations of an acoustic window, is highly effective and reproducible for the assessment of RV volume and function in patients with LVADs (52).
CTA may also be able to provide more than just structural data for LVADs. Dynamic CTA with mathematic analyses of flow can be used to determine cardiac output in patients with a continuous-flow LVAD. In one study, CTA measurements of cardiac output in the ascending aorta distal to the anastomosis of the outflow cannula correlated with cardiac output measurements by Swan–Ganz thermodilution during right heart catheterization (53). Although cardiac output calculation by CTA is a potentially exciting future application of CTA for patients with LVAD, the clinical utility of this modality remains to be determined.
Heart Transplant Vasculopathy
Heart transplantation remains the paramount treatment modality for end-stage heart failure, with a 5-y survival of approximately 70% (54). However, after heart transplantation, one of the most feared complications is cardiac allograft vasculopathy (CAV), consisting of concentric and diffuse coronary intimal hyperplasia. CAV affects up to 50% of transplant recipients within 10 y, and those with severe CAV have a poor prognosis (55). Therefore, routine screening for CAV after transplantation is necessary, especially given its absent or atypical symptomatology.
The diagnosis of CAV has traditionally relied on the use of coronary angiography. Intravascular ultrasound during ICA is the most sensitive method for detecting CAV because it can measure intimal thickening within the coronary artery wall itself (56). Many noninvasive testing modalities, including exercise electrocardiography, stress echocardiography, and myocardial perfusion imaging, are limited by modest diagnostic accuracy and are not recommended as screening tools for CAV (57). However, CTA is safe and feasible for monitoring heart transplant recipients (58), and it provides the theoretic advantage over traditional ICA of being able to visualize both the lumen and the vessel wall simultaneously. A recent metaanalysis (59) demonstrated that modern CTA is a reliable noninvasive imaging alternative for the detection of CAV, even in comparison with intravascular ultrasound, with excellent sensitivity, specificity, and negative predictive value.
LIMITATIONS
Despite the many advantages of CTA for patients with heart failure, it is important to delineate the limitations of and risks associated with CTA. First and foremost, cardiac CT requires the use of intravenous iodinated contrast material. Beyond the small incidence of an anaphylactic reaction, there is the known risk of nephrotoxicity, which is especially important for patients with heart failure because many patients with end-stage disease have concomitant renal impairment. Second, patients are required to hold their breath for up to 10 s during image acquisition for CTA. Although this requirement is almost always feasible, it can pose a problem for a patient with decompensated heart failure because such a patient may not be able to perform an adequate breath hold. Another issue is the need for low and regular heart rates, which can directly affect the quality of the study. Patients with heart failure often have frequent premature ventricular contractions, and, as discussed earlier, atrial fibrillation is a common comorbidity in such patients. Finally, CTA involves radiation exposure. The effective radiation dose of CTA has progressively declined to the 1- to 5-mSv range, depending on whether the study is prospectively or retrospectively gated (60); diagnostic ICA delivers a mean effective radiation dose of approximately 2–2.5 mSv (61). Dose reduction techniques continue to be improved, further reducing radiation exposure, but the risks of higher radiation doses must be considered in decisions about the appropriate testing modality for a particular patient.
CONCLUSION
CTA has great utility in the domain of clinical heart failure. For diagnosing heart failure, differentiating between etiologies, and planning and monitoring various treatment modalities, CTA provides a quick, noninvasive, and comprehensive analysis of native cardiac and implanted device structure and function as well as a detailed evaluation of both arterial and venous vasculature. Moreover, with the ongoing development and advancement of noninvasive flow calculations, CTA may be able to provide a hemodynamic assessment of heart failure pathophysiology in the future.
DISCLOSURE
Harvey S. Hecht is a Philips Medical Systems Consultant. No other potential conflict of interest relevant to this article was reported.
- © 2015 by the Society of Nuclear Medicine and Molecular Imaging, Inc.
REFERENCES
- Received for publication January 6, 2015.
- Accepted for publication March 27, 2015.