Abstract
Recent studies have shown that idiopathic atrial fibrillation (AF) is associated with diminished myocardial perfusion and perfusion reserve, which are also impaired in various forms of cardiomyopathies. In many cases, AF develops during progression of dilated cardiomyopathy (DCM) and may aggravate heart failure. This study compared myocardial perfusion between patients with nonischemic DCM with and without AF. Methods: Twelve men (age ± SD, 55 ± 12 y) who had DCM and persistent AF were compared with a group of 18 men (mean age, 43 ± 15 y, P = not statistically significant) who had DCM and sinus rhythm and with 22 healthy controls (mean age, 47 ± 13 y, P = not statistically significant). Myocardial blood flow (MBF) was noninvasively quantified at rest and during adenosine infusion using PET and radioactive-labeled water (H215O PET). Results: Compared with controls, DCM patients without AF showed impaired hyperemic perfusion (2.52 ± 1.29 vs. 3.57 ± 0.88 mL/min/mL, P = 0.014) and perfusion reserve (2.10 ± 1.01 vs. 3.37 ± 0.97, P = 0.003). However, compared with DCM patients without AF, DCM patients with AF showed an additional impairment in resting perfusion (0.82 ± 0.31 mL/min/mL, P = 0.010) and hyperemic perfusion (1.32 ± 0.93 mL/min/mL, P = 0.022), and compared with controls, DCM patients with AF showed a further diminishment of perfusion reserve (1.68 ± 0.94 vs. 3.37 ± 0.97, P < 0.001) accompanied by the highest coronary vascular resistance of all groups. Conclusion: Compared with patients with sinus rhythm, patients with AF have significantly reduced myocardial perfusion reserve and increased coronary resistance in nonischemic DCM. Further studies on the underlying pathomechanisms are warranted.
Atrial fibrillation (AF) is the most common sustained arrhythmia in humans. It frequently requires medical treatment and is associated with increased morbidity and mortality (1). We have recently shown that idiopathic AF is associated with diminished myocardial perfusion and perfusion reserve (2). However, AF is more likely to develop on the basis of a structural heart disease and occurs in more than a quarter of cases with dilated cardiomyopathy (DCM) (3). Many patients with nonischemic DCM, as well as patients with idiopathic AF, present with symptoms suggestive of myocardial ischemia, such as unspecific retrosternal sensations resembling angina pectoris, despite exclusion of coronary artery disease. In nonischemic DCM, elevated markers of myocardial damage have been reported after cardiac decompensation (4). AF on top of an existing cardiomyopathy further impairs both cardiac function and individual prognosis and leads to an increased number of hospitalizations due to congestive heart failure (5). Restoration of sinus rhythm (SR) in patients with congestive heart failure and AF may ameliorate ventricular function and may spare heart transplantation (6). Myocardial perfusion and perfusion reserve are known to be impaired in various forms of cardiomyopathy (ischemic and nonischemic) even without clinically overt congestive heart failure (7).
On the basis of these facts, we hypothesized that myocardial perfusion and perfusion reserve could be less in patients with nonischemic DCM and additional AF than in patients with DCM in SR. This impairment could have prognostic and future therapeutic implications, especially since the level of hyperemic perfusion has been shown to be an independent predictor of prognosis in patients with DCM (8). Therefore, our study investigated the difference in myocardial perfusion and perfusion reserve in patients with nonischemic DCM with and without AF using noninvasive PET and radioactively labeled water (H215O PET).
MATERIALS AND METHODS
Study Population
Patients.
A total of 30 male DCM patients were enrolled in this study. DCM was clinically diagnosed on the basis of reduced ventricular function (ejection fraction < 55%) with left ventricular dilatation in the absence of coronary artery disease (9). Coronary artery disease was excluded on the basis of absence of clinical symptoms suggestive of ischemia, absence of signs of ischemia in exercise electrocardiograms, and additional coronary angiography studies in 28 of 30 DCM patients and stress myocardial SPECT studies in the remaining 2 of 30. Further exclusion criteria were other functional cardiac diseases, hyperthyroidism, and any grade of valvular stenosis except for valvular regurgitation lower than grade 2.
Within the DCM group, 12 patients (mean age, 55 ± 12 y) had persistent AF (mean duration, 32 ± 39 mo) (DCM/AF group), whereas the remaining 18 patients (mean age, 43 ± 15 y, P = not statistically significant [NS] vs. DCM/AF) showed stable SR and no history of AF (DCM/SR group). None of these patients was included in our prior study (2) of patients with idiopathic AF and otherwise healthy hearts, and there is no overlap between these study groups.
Three patients in the DCM/AF group and 5 patients in the DCM/SR group had implanted cardioverter-defibrillators because of a history of life-threatening tachyarrhythmias. None of the devices worked in pacemaker mode during the scan or beforehand, as ensured by interrogation of the device.
Most patients in both DCM groups were in New York Heart Association functional class II or III (2.06 ± 1.07 for DCM/SR vs. 2.40 ± 0.52 for DCM/AF, P = NS).
Echocardiography was performed on all patients by expert investigators to assess atrial and ventricular dimensions and ventricular and valvular function (Table 1). These parameters were quantified in several consecutive measurements in diastole for an average R-R interval as measured by continuous monitoring during echocardiography. This technique is in line with former study protocols (10).
Study Population: Characteristics and Medication
Between the DCM/SR and DCM/AF groups, echocardiographically assessed left ventricular diameters, and function did not show any significant difference. As expected, the diameter of the left atrium was larger in the DCM/AF patients than in the DCM/SR patients and controls.
The clinical characteristics of DCM/AF and DCM/SR patients are detailed in Table 1; echocardiographic parameters are listed in Table 2.
Echocardiographic Parameters
Control Subjects.
Twenty-two healthy male control subjects (mean age, 47 ± 13 y, P = NS vs. DCM/AF and DCM/SR) who had SR were enrolled. Coronary artery disease was excluded as described above and additionally by invasive angiography in 13 of 22 subjects (59%), proving that there were no signs of coronary artery disease. All control subjects underwent maximal exercise stress testing before inclusion in the study, without any pathologic findings. Clinical and echocardiographic data are given in Tables 1 and 2.
Study Design
Noninvasive Measurement of Myocardial Perfusion In Vivo.
PET scans were obtained after the subjects had fasted for at least 12 h, particularly excluding caffeine-containing beverages, chocolate, and smoking to avoid any interference with adenosine. All medications were withheld for 24 h before imaging. All scans were initiated between 8:00 and 9:00 am, assuring the same circadian conditions in all participants. Before baseline measurements, all study participants rested supine on the scanner for 15 min.
The study followed a protocol previously published (2). Briefly, myocardial blood flow (MBF) was assessed by dynamic PET (ECAT-921; Siemens/CTI) after an intravenous bolus injection of 500 MBq of H215O over 20 s. A 26-frame dynamic PET acquisition was obtained over 5 min. The emission data were reconstructed (Hanning filter, 7.3 mm in full width at half maximum, zoom factor of 2.3, 47 planes, and matrix size of 128 × 128). Factor images were generated from the dynamic H215O scans and resliced into short-axis images perpendicular to the long axis of the left ventricle. This transformation matrix was also used for reslicing the dynamic water images. Regions of interest (ROIs) were placed manually on the short-axis planes of the factor images encompassing left ventricular myocardial tissue, the left atrial cavity, and the right ventricular cavity. The myocardial ROIs covered the whole left ventricular myocardium since these were drawn on all short-axis images showing myocardium, typically 11–12 slices. In addition to the assessment of a single ROI comprising the whole left ventricular myocardium, separate values were obtained for 4 ROIs by dividing the myocardial tissue ROI into an anterior, lateral, inferior, and septal region.
Arterial, venous, and tissue time–activity curves were fitted to a single-compartment model to quantify regional and global MBF (mL/min/mL) and perfusable tissue fraction (milliters of water-perfused tissue per milliliter of ROI) (11).
All patients and control subjects gave written informed consent to the study protocol, which was approved by the Ethics Committee of the Medical faculty of the University of Münster and the Chamber of Physicians (Ärztekammer Westfalen-Lippe), Münster, Germany.
Hyperemic Flow and Flow Reserve
Additionally, hyperemic MBF was measured from a second injection of H215O 2 min after initiation of a 7-min adenosine infusion at 140 μg/kg of body weight per minute. The hyperemic coronary flow reserve was calculated as the ratio of hyperemic and baseline MBF, the latter represented by the MBF corrected for the rate–pressure product (RPP). To allow for sufficient decay of the radioactivity from the first PET scan, the adenosine PET scan started 20 min after the end of the resting scan.
Hemodynamics
Heart rate and systolic, diastolic, and mean arterial blood pressures were determined for each subject during PET scans. Because of the unreliability of heart rate detection by a bedside electrocardiography monitor in AF, caused by beat-to-beat R-R interval changes, mean heart rates and variance of cycle length were calculated from continuous Holter recordings during the scans (R-R intervals SD of normal to normal).
Coronary Vascular Resistance (CVR)
CVR in the different settings (baseline and adenosine) was calculated by dividing mean arterial pressure by the respective MBF.
Statistical Analysis
Results are expressed as mean ± SD. After testing for the equality of variances (Levene test), ANOVA in connection with the Bonferroni correction was performed for comparisons of global values of MBF, CVR, and coronary flow reserve between groups. The Pearson correlation coefficient was calculated to investigate associations between possible confounders and measured data of perfusion, perfusion reserve, and CVR. A P value of less than 0.05 was considered significant.
RESULTS
Hemodynamics
Data on hemodynamics are given in Table 3. To eliminate potential interindividual differences in cardiac workload that could influence MBF, MBF at rest was corrected for the rate–pressure product (MBFcorrected = MBF/RPP × 10,000) (12). Additionally, all values of uncorrected MBF at baseline are also listed in the text and in Figure 1. Adenosine-induced MBF remained uncorrected because the MBF increase stimulated by adenosine is grossly independent of RPP (13).
MBF in DCM/AF, DCM/SR, and controls as measured by H215O PET at baseline and under adenosine infusion (ADO). MBF at baseline has been corrected for rate–pressure product.
Hemodynamics
MBF and Flow Reserve
DCM/SR Versus Controls.
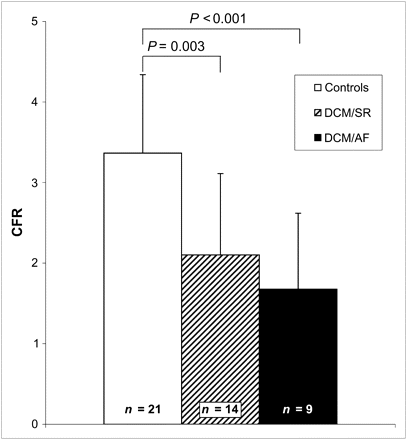
As expected from previously published studies (14), resting MBF corrected for RPP was comparable in DCM/SR patients and controls (1.13 ± 0.31 vs. 1.14 ± 0.20 mL/min/mL, P = NS). These similarities were consistently found in MBF values uncorrected for the rate–pressure product (0.84 ± 0.27 vs. 0.87 ± 0.16 mL/min/mL, P = NS) (Fig. 1). In contrast, hyperemic MBF (2.52 ± 1.29 vs. 3.57 ± 0.88 mL/min/mL, P = 0.014) and hyperemic coronary flow reserve (2.10 ± 1.01 vs. 3.37 ± 0.97, P = 0.003) were significantly impaired in DCM/SR patients (Figs. 1 and 2), compared with controls.
Coronary flow reserve (CFR) calculated from MBF under adenosine infusion divided by baseline MBF corrected for rate–pressor product in DCM/AF, DCM/SR, and controls as determined by H215O PET.
DCM/AF Versus DCM/SR.
Interestingly, baseline MBF corrected for RPP was significantly lower in DCM/AF patients (0.82 ± 0.31 mL/min/mL, P = 0.010) than in DCM/SR patients. Accordingly, uncorrected baseline MBF was also lower in DCM/AF patients and significantly less than in controls (0.67 ± 0.20 mL/min/mL, P = 0.038 vs. controls). Even more pronounced, hyperemic MBF was further impaired in DCM/AF patients (1.32 ± 0.93 mL/min/mL, P = 0.022) (Fig. 1). As a consequence, DCM/AF patients showed an almost blunted hyperemic coronary flow reserve (1.68 ± 0.94 mL/min/mL, P = NS) (Fig. 2).
Regional MBF
In addition to these global analyses of MBF, regional MBF in 4 ROIs (anterior, lateral, inferior, and septal walls) was analyzed. Myocardial perfusion was homogeneously distributed throughout the left ventricular myocardium in all groups. None of the regions showed a perfusion significantly different from any other region within any single group. Global perfusion differences between groups did not show any dependency on specific regional distribution patterns. Table 4 contains a detailed list of regional MBF values.
Regional Myocardial Perfusion
CVR
DCM/SR Versus Controls.
CVR was similar in DCM/SR patients and controls at baseline (106 ± 34 vs. 109 ± 21 mm Hg × mL−1 × min × mL, P = NS) and under adenosine infusion (49 ± 51 vs. 27 ± 7 mm Hg × mL−1 × min × mL, P = NS) (Fig. 3).
CVR at baseline and under adenosine administration (ADO) in DCM/AF, DCM/SR, and controls.
DCM/AF Versus DCM/SR.
In DCM patients, the presence of AF dramatically increased CVR at baseline (150 ± 40 mm Hg × mL−1 × min × mL, P = 0.001) and under adenosine infusion (95 ± 45 mm Hg × mL−1 × min × mL, P = 0.013) (Fig. 3).
Clinical Characteristics and Potentially Confounding Factors
Age and Sex.
Only male subjects were studied. The mean age of DCM/AF patients was not significantly different from that of DCM/SR patients.
Risk Factors.
The DCM groups did not significantly differ with respect to any single coronary risk factor (total cholesterol, high LDL cholesterol, low HDL cholesterol, hypertension, diabetes, smoking, or family history of coronary artery disease) or the Framingham score assessing the individual global cardiovascular risk. No correlation between these risk factors or the risk score and PET data was found within any group.
DISCUSSION
AF in the presence of structural heart disease is a frequent clinical problem (3) that may severely affect the patients' quality of life and cardiac function (5,6). In a recent study, we found impaired myocardial perfusion and perfusion reserve in idiopathic AF (2). Therefore, the aim of this study was to investigate whether the presence of AF is also associated with impairment of myocardial perfusion in patients with nonischemic DCM. We have used state-of-the-art PET technology uniquely able to quantify myocardial perfusion and perfusion reserve in vivo in DCM patients with SR or persistent AF. The main and new findings of this study are that, compared with DCM patients with preserved SR, patients with DCM and persistent AF show markedly decreased myocardial perfusion at rest and under hyperemia whereas CVR is increased.
Resting Perfusion
In comparing DCM patients with SR versus controls, we found that the underlying structural heart disease alone did not affect resting MBF or CVR. However, in DCM patients with AF, the resting MBF corrected for RPP was significantly diminished by about 27% whereas the CVR was elevated by about 42%. This finding is novel, since other techniques previously investigating perfusion in patients with DCM and AF could not quantify baseline MBF and CVR in absolute terms. The findings are in line with, yet exceed, the former finding of an approximately 20% reduction of resting MBF corrected for RPP in idiopathic AF (2).
Resting Perfusion Uncorrected for Rate–Pressure Product
Uncorrected MBF was about 20% lower in patients with DCM and additional AF than in patients with DCM in SR. This finding underlines that differences in myocardial perfusion at baseline do not occur secondary to the correction for the rate–pressure product but already exist in absolute uncorrected data. The fact that correcting for the respective cardiac workload sharpens the contrast between DCM patients with and without AF significantly due to a higher rate–pressure product in DCM/AF patients points to the insufficient pathophysiologic compensatory mechanism. Patients with DCM and additional AF achieve lower myocardial perfusion despite a simultaneously higher cardiac workload than that in DCM patients in SR. Therefore, correction of the MBF at baseline for the rate–pressure product adds relevant information since it reveals the whole dimension of the diminished myocardial perfusion in contrast to a simultaneously elevated (ineffective) cardiac workload in patients with DCM and AF.
Regional Perfusion Distribution
Our study did not show any divergent regional perfusion patterns in patients with DCM and additional AF, compared with SR. The diminished myocardial perfusion in the DCM/AF group prevails equally in all regions of the heart. This finding supports the hypothesis of a global pathophysiologic mechanism diminishing perfusion in all myocardial regions simultaneously and equally. A regionally localized influencing factor can merely be excluded from these data. This is in line with many mechanistic studies describing systemically changed vasoconstrictive tones in AF.
Hyperemic Perfusion
Under adenosine infusion, DCM patients with additional AF reached only about 37% of the MBF measured in controls and about 52% of the MBF in DCM patients without AF. In comparing DCM patients without AF versus controls, we found that the underlying DCM seemed to have an independent effect on hyperemic MBF, in line with earlier studies (14). However, hyperemic perfusion in DCM patients with AF is massively diminished and does not exceed even levels of normal resting flow within controls. Accordingly, minimal CVR under adenosine was twice as high in DCM patients with AF as in DCM patients without AF. The presence of AF in DCM patients was associated with a degree of perfusion impairment exceeding that found in otherwise healthy hearts (1) or in valvular (15) and artificially induced acute AF (16). To our knowledge, impairments of such an extent in absolute hyperemic perfusion are unparalleled in the literature so far.
Pathophysiologic Considerations
This study design was, for the first time, able to show perfusion differences between DCM patients with and without AF but prevented us from examining pathophysiologic considerations. This ability would require an interventional design, such as before and after electrical cardioverter therapy. Theoretically, our observations could be attributed to the tachyarrhythmia itself. Besides the acceleration in heart rate, the irregularity of ventricular cycle lengths in AF may be disadvantageous for hemodynamics (17) and overall cardiac function. Intraindividual reduction of variance of cycle length by pacing (18) or cardioversion to SR (19) reameliorated these functional deficits. Still, there was no interindividual correlation between variance of cycle length and myocardial perfusion within our DCM/AF population.
The diminished perfusion was accompanied by an elevation in CVR. This elevation might indicate a shift toward an increased vasoconstrictive tone. Former studies support the hypothesis of a systemically mediated vasoconstriction in AF, showing that forearm vessels also lose vasodilator capacity during AF (20). In DCM patients, however, perfusion abnormalities appear to be restricted to the microvasculature without afflicting larger vessels (21).
Potential mediators of vasoconstriction might be of neurohumoral origin, as suggested by raised plasma levels of atrial natriuretic peptide or brain natriuretic peptide under AF (22), as well as their renormalization after successful catheter ablation of AF (23). Additionally, raised levels of the angiotensin-converting enzyme have been found in chronic AF (24).
A second component might be an elevated sympathetic tone (25) with a reamelioration of hyperemic flow reserve after administration of an α1-adrenoceptor antagonist (26). A change in adrenergic tone was also observed for an underlying DCM (27).
Furthermore, the arrhythmia might even join forces with the underlying structural heart disease and remodel the myocardium. Evidence of negative remodeling in AF has been supplied (28), as well as proof of its iterative reversibility (29).
To elucidate how far these observations of impaired myocardial perfusion correlate with outcome in patients with underlying DCM and additional AF, and whether restoration of SR can reameliorate myocardial perfusion, was not in the scope of this study and requires further longitudinal and interventional studies.
Limitations
This study describes, for the first time, impaired perfusion reserve and increased coronary resistance in DCM and AF. To study whether these changes are associated with, or independent of, the arrhythmia, one could individually reanalyze patients (second PET series) after restoration of SR. However, the patients in this study were recruited on the basis of proven nonischemic DCM. In none of the patients was restoration of SR by electrical cardioverter therapy planned or feasible. This limitation prevents us from drawing the respective pathophysiologic conclusion.
Statistical proof that AF diminishes myocardial perfusion as an independent factor with the help of a multivariate analysis could not be provided because of the small patient sample in combination with a broad variety of potentially confounding factors.
Patients with DCM/AF were slightly older than DCM patients without AF. However, when tested, this finding was not statistically significant. Furthermore, New York Heart Association functional classes and echocardiographic findings were comparable between the 2 groups; DCM patients therefore seem to be well comparable between the groups.
Although control subjects showed a tendency toward higher cardiovascular risk as assessed by analysis of risk factors known for their detrimental effects on myocardial perfusion, there was no statistically significant difference between the 2 DCM groups. However, this could result only in lower differences between controls and DCM patients and does not explain the findings of the study. Because of the relatively small sample size, potential effects of digitalis could not be analyzed statistically. There have been hints that cardiac glycosides (especially ouabain) cause acute vasoconstriction that fades within minutes (30). In contrast, for digoxin and digitoxin, which were given to our patients, vasodilatory effects have been discussed (31). In early invasive studies, the resulting absolute global MBF was found to be unchanged by cardiac glycosides (32). Especially in the long-term treatment of failing human hearts, beneficial effects on coronary circulation can be expected, as concluded in one study (33). Therefore, it is unlikely that the effects of digitalis contributed to our findings of diminished perfusion in additional AF.
CONCLUSION
Patients with nonischemic DCM and persistent AF, compared with those having SR, show significantly diminished myocardial perfusion and perfusion reserve and increased CVR. Further studies on the pathophysiologic interrelation between the arrhythmia and this functional finding are warranted.
Acknowledgments
We gratefully acknowledge the assistance and support of Silke Schroer and of the PET Centre Group. This study was supported by grants from the Interdisciplinary Centre of Clinical Research Münster (IZKF, project B10) and from the Deutsche Forschungsgemeinschaft (DFG SFB 656, projects A1 and C2).
Footnotes
-
↵* Contributed equally to this work.
-
COPYRIGHT © 2009 by the Society of Nuclear Medicine, Inc.
References
- Received for publication July 3, 2008.
- Accepted for publication December 5, 2008.