Abstract
Cell therapy–induced changes in the perfusion of areas of myocardial infarction (MI) remain unclear. This study investigated whether an original pinhole SPECT technique could be applied to a rat MI model to analyze local improvement in myocardial perfusion relating to engraftment sites of bone marrow–derived stem cells (BMSCs). Methods: Four-month-old MI rats were either untreated (n = 8) or treated (n = 10) by intramyocardial injection of 111In-labeled BMSCs. Early distribution of 111In-BMSCs within the MI target was evidenced by dual 111In/99mTc pinhole SPECT 48 h later. Myocardial perfusion was serially monitored by 99mTc-sestamibi pinhole gated SPECT up to 3 mo after transplantation. Results: Forty-eight hours after transplantation, 111In-BMSCs were observed in all treated rats and in 18 of their 32 underperfused MI segments (<70% sestamibi uptake before transplantation). During the subsequent 3-mo follow-up, the perfusion of MI segments worsened in untreated rats (absolute change in sestamibi uptake, −3% ± 3%; P < 0.05) but improved in treated rats (+4% ± 7%; P < 0.05). This perfusion improvement was unrelated to the initial detection of 111In-BMSCs (+2% ± 6% in segments with 111In-BMSCs vs. +5% ± 7% in those without; not statistically significant) but was strongly associated with less severe perfusion defects before transplantation (+6% ± 6% in segments with 60%–70% sestamibi uptake [n = 19] vs. −1% ± 6% in those with <60% uptake [n = 13]; P = 0.003). Conclusion: When BMSCs are injected within chronic MI, perfusion enhancement predominates in the MI areas showing a high enough residual perfusion before treatment but not in those of the initial cell engraftment, giving evidence of dependency on the perfusion and metabolic environment at implantation sites.
- myocardial infarction
- rats
- stem cell therapy
- bone marrow mesenchymal stem cells
- sestamibi
- 111In-oxine
- pinhole SPECT
In humans, coronary occlusion persists after myocardial infarction (MI) and may lead to a progressive extension of infarcted areas and to fibrous development (1). This deleterious remodeling may be slowed by the reperfusion of infarcted tissue, even when the reperfusion occurs belatedly (2). Attempts are being made to enhance perfusion in areas of MI using direct implantation of bone marrow–derived stem cells (BMSCs). This therapy relies on the intrinsic ability of these cells to promote neoangiogenesis (3,4) and cardiomyocyte development (5,6), although the latter point is more controversial than the former.
Histopathologic analyses of infarcted areas treated by BMSCs have consistently documented a dramatic increase in the density of microvessels (7–9), but the effect on local variations of myocardial perfusion has remained unclear. In particular, it is not known whether myocardial perfusion improves at the specific sites of cell implantation and whether any improvement is influenced by local metabolic factors such as the baseline levels of perfusion and viability. An understanding of these issues would allow the definition of optimal conditions for cell implantation.
We recently developed an original pinhole-SPECT technique providing 3-dimensional images of 99mTc-sestamibi uptake in rat myocardium (10–12) similar to those already provided by conventional SPECT in humans (13,14). In the present study, this technique was used, first, to determine the baseline level of perfusion within chronically infarcted myocardial segments in rats; second, to precisely localize the sites of early retention of 111In-labeled BMSCs after they have been injected within the infarcted areas; and third, to assess relationships with the subsequent regional improvement in myocardial perfusion.
MATERIALS AND METHODS
Animal and General Protocols
Eighteen male Wistar rats, weighing 410–460 g at the beginning of the study, were used. All experimental procedures were in accordance with our local ethical committee and with the regulations of the Animal Welfare Act and with the National Institutes of Health Guide for the Care and Use of Laboratory Animals (15).
Four months after surgical occlusion of the left anterior descending artery (LAD), 10 rats were assigned to receive cell therapy, whereas the other 8 were not. Cell therapy was performed using autologous bone marrow mesenchymal stem cells (BMSCs), which were labeled with 111In-oxine (16), and a unique dose of 111In-BMSCs (2 × 106 cells) was directly injected within the infarcted areas. Two days later, dual 111In/99mTc-sestamibi pinhole SPECT was performed to localize sites of early retention of 111In-BMSCs (12).
The underperfused area of MI and the left ventricular (LV) function were monitored in all treated and untreated animals by serial 99mTc-sestamibi pinhole gated SPECT performed before cell therapy (at 1 and 3 mo after MI) and after cell therapy (at 5 and 7 mo after MI).
Rat MI Model
The protocol of coronary occlusion has been described previously (17). Briefly, all rats were anaesthetized with ketamine (100 mg/kg, intramuscularly) and diazepam (5 mg/kg, intraperitoneally) and mechanically ventilated. The heart was exposed through a 1.5-cm lateral thoracotomy of the fifth interrib space. After pericardial incision, the LAD artery was ligated by means of a 7/0 polypropylene suture.
Culture, Labeling, and Transplantation of Mesenchymal Cells
Autologous bone marrow was harvested through a tibial puncture, and BMSCs were cultured for up to 2–3 wk as previously described (17). Briefly, aspirated bone marrow cells were suspended in Iscove's modified Dulbecco's culture medium (Gibco Laboratory, Life Technologies) containing 10% fetal bovine serum (Gibco Laboratory, Life Technologies), 0.1 mmol of β-mercaptoethanol per liter (Sigma), 100 U of penicillin per milliliter, and 100 μg of streptomycin per milliliter. Cells were grown in a 5% humidified CO2 atmosphere at 37°C, the medium being changed every 2 d.
Before transplantation, the cultured cells were trypsinized and incubated at 37°C with 15 MBq of 111In-oxine (Mallinckrodt Medical B.V.) during a 10-min period (2 × 106 cells per mL), the labeling process being stopped by a 5-min centrifugation at 950g. This 10-min incubation period was previously found to result in sufficient labeling efficiency (69%) without significant deterioration of cell viability (96%) (16).
The 111In-labeled cells were conditioned in an insulin syringe (2 × 106 cells in 50 μL), and a single injection was performed through a mini thoracotomy redux, within the core of the visually delineated fibrotic area of MI, the animals being anaesthetized and mechanically ventilated using a method similar to that used for LAD ligation.
Pinhole SPECT Acquisition
In all treated and untreated animals, serial 99mTc-sestamibi pinhole gated SPECT was performed according to a previously described method (10,11) using a conventional single-head γ-camera (DSX; GE Healthcare–SMV) equipped with a 3-mm pinhole collimator. The animals were previously sedated by an intraperitoneal injection of 60 mg of sodium pentobarbital per kilogram of body weight, and acquisitions began 40–60 min after 400–700 MBq of 99mTc-sestamibi had been intravenously injected. Thirty-two projections of 30 s each were acquired on a 180° circular orbit centered on the heart (from 110° right anterior to 290° left anterior) using the following parameters: a 64 × 64 matrix, a 2.66 zoom, 16 bins, a 140% ± 20% keV energy window, and the beat acceptance window set to ±20% of the averaged R-R interval. The total acquisition time was approximately 20 min.
In the 10 animals who received cell therapy, an additional dual-energy pinhole SPECT study was acquired 2 d after therapy, 1 h after the intravenous injection of 200 MBq of 99mTc-sestamibi and using a method similar to that used for the serial 99mTc-sestamibi gated SPECT studies, except that acquisition was ungated and involved separate recording of the activities from 99mTc (140% ± 20% keV energy window) and 111In (172% ± 15% keV and 246% ± 15% keV energy windows).
Reconstruction and Analysis of Pinhole SPECT Images
All gated and ungated data were corrected for the mechanical shift of camera-head rotation and for the nonuniform sensitivity of pinhole detection, according to a previously described method (10,18,19). Images were thereafter reconstructed using an ordered-subset expectation maximization iterative process (2 iterations and 8 subsets) adapted to take into account the pinhole geometry. For gated SPECT reconstructions, temporal Fourier filtering was additionally incorporated within ordered-subset expectation maximization reconstructions.
Standard clinical software (Mirage cardiogram processing application; Segami) was used for reorientation along the LV long axis of all gated and ungated images and for providing quantitative polar-map displays of tracer distribution on ungated images, according to the standard 17-segment division of the left ventricle, the 2 proximal septal segments being excluded (membranous septal wall).
The LV segments showing BMSC retention were identified visually on a paired polar-map display of the LV activities from 111In-BMSCs and 99mTc-sestamibi (12). The LV segments, corresponding to the underperfused MI area, were called MI segments and were defined as those showing a 99mTc-sestamibi uptake lower than 70% of the maximum voxel value on the sestamibi pinhole SPECT study obtained before transplantation (3-mo-old MI). This threshold value of 70% was previously found to correspond to the mean segmental value − 1.5 SDs for sestamibi uptake in 9 normal adult rats (12).
LV end-diastolic and end-systolic volumes, as well as LV ejection fraction, were determined automatically by quantitative gated SPECT software (QGS; General Electric Co.) on the contiguous gated short-axis slices obtained from serial 99mTc-sestamibi gated SPECT.
Statistics
Quantitative variables were expressed as mean ± SD, and they were compared using nonparametric tests: Mann–Whitney tests for unpaired comparisons between treated and untreated animals and Wilcoxon tests for paired comparisons in each group. Regression linear analyses were also applied to the MI segments from treated rats (those showing <70% sestamibi uptake on pretherapeutic pinhole SPECT) to determine predictors of the absolute changes in 99mTc-sestamibi uptake at 3 mo after transplantation. For each test, a P value of less than 0.05 was considered to indicate a significant difference.
RESULTS
LV Function and Perfusion Before Cell Therapy
Pinhole gated SPECT performed 1 mo before cell therapy and 3 mo after MI showed a highly variable underperfused area of MI involving 1–7 LV segments (5%–50% of the left ventricle). Consequently, large ranges of LV end-diastolic volume (209–422 μL) and of LV ejection fraction (39%–72%) were also documented. As detailed in Table 1, all quantitative parameters of LV function and perfusion were equivalent between the rats that subsequently underwent cell therapy and the untreated control rats.
Comparison of Quantitative Variables Between Untreated Rats and Treated Rats
Initial Sites of Engrafted 111In-BMSCs
Dual-energy pinhole SPECT performed 48 h after cell transplantation detected 111In-labeled BMSCs in 37 segments (1–3 segments per rat): 18 (49%) had been identified as underperfused MI segments on the pretherapeutic pinhole SPECT, 18 were adjacent to such MI segments, and 1 was a remote segment. Finally, 111In-labeled BMSCs were not detected in 14 (44%) of the 32 segments identified as underperfused MI segments before therapy.
Posttherapeutic Evolution of Global LV Function and Perfusion
As detailed in Table 1, both treated and untreated rats exhibited LV enlargement, as well as a decrease in LV ejection fraction, between pretherapeutic pinhole SPECT and SPECT performed at the end of follow-up—that is, 7 mo after MI. Compared with untreated rats, however, treated rats had lower LV enlargement rates during this period, with a lower relative increase in end-diastolic volume (+6% ± 6% vs. +22% ± 16%, P = 0.03), leading to a clear trend toward smaller absolute values of end-diastolic volume at the end of follow-up (P = 0.06; Table 1).
Rats from the treated group, but not those from the untreated group, showed a significant reduction in the number of underperfused segments between pretherapeutic SPECT and that recorded at the end of follow-up (Table 1). Consequently, this number became lower in treated than in untreated animals at the end of follow-up, even though the difference was only of borderline statistical significance (P = 0.05; Table 1).
Posttherapeutic Evolution of Segmental Perfusion
As detailed in Figure 1A, during the 3 mo after cell injection, the perfusion of infarcted segments worsened in untreated rats (absolute change in sestamibi uptake: −3% ± 3%, P < 0.05; Fig. 2) and improved in treated rats (+4% ± 7%, P < 0.05; Figs. 3 and 4), and no perfusion change was documented within noninfarcted segments for either treated or untreated rats.
Evolution of myocardial perfusion, as assessed by sestamibi uptake, in the analyzed segments overall (A), in MI segments only (B), and in MI segments from treated rats only (C). (A) Segments were classified as belonging (circles) or not belonging (squares) to underperfused areas (≤70% uptake on pretherapeutic SPECT) and were compared between treated rats (n = 118 [□] and 32 [○]) and untreated rats (n = 95 [▪] and 25 [•]). (B) Segments were classified as having (triangles) or not having (diamonds) severe perfusion defects (<60% uptake) before transplantation and were compared between treated rats (n = 19 [⋄] and 13 [▵]) and untreated rats (n = 13 [♦] and 12 [▴]). (C) Segments were classified as having (triangles) or not having (diamonds) severe perfusion defects (<60% uptake) before transplantation and were compared between rats that had (n = 10 [♦] and 4 [▴]) and rats that did not have (n = 8 [⋄] and 10 [▵]) early retention of 111In-BMSCs. *P < 0.05 for unpaired comparisons. TT= treated; UT = untreated.
99mTc-Sestamibi pinhole SPECT images of untreated rat 1, 3, 5, and 7 mo after MI. Perfusion defects clearly worsened over time. A = anterior wall; I = inferior wall; L = lateral wall; and S = septal wall.
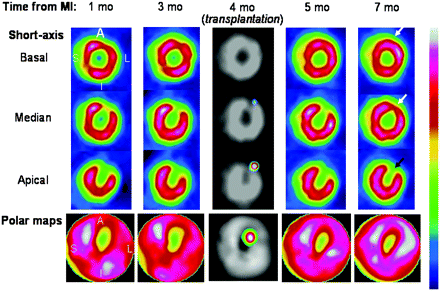
99mTc-Sestamibi pinhole SPECT images of treated rat 1, 3, 5, and 7 mo after MI and, in center column, dual 111In/99mTc-sestamibi pinhole SPECT images 48 h after transplantation. Perfusion improved in areas that had only moderate defects before treatment (around 60% uptake), both at implantation site (black arrow) and at neighboring site (white arrow). A = anterior wall; I = inferior wall; L = lateral wall; and S = septal wall.
99mTc-Sestamibi pinhole SPECT images of treated rat 1, 3, 5, and 7 mo after MI and, in center column, dual 111In/99mTc-sestamibi pinhole SPECT images 48 h after transplantation. No clear improvement in perfusion was seen in area that had severe defects before treatment (<50% uptake; black arrow) and where the greatest number of implanted cells had been detected. By contrast, improvement of perfusion was clear in neighboring area that had less severe defects before treatment (white arrows). A = anterior wall; I = inferior wall; L = lateral wall; and S = septal wall.
The improved perfusion in MI segments from treated rats varied considerably, however, with absolute changes in sestamibi uptake ranging from −10% to +20% at the end of follow-up. A case of no improvement in sestamibi uptake is shown in Figure 5. Moreover, improved perfusion did not predominate in the segments where 111In-BMSCs had been detected (absolute change in sestamibi uptake between pretherapeutic pinhole SPECT and SPECT at the end of follow-up: +2% ± 6%), when compared with the other MI segments from treated rats (+5% ± 7%, not statistically significant). By linear regression analysis, finally, this variable perfusion improvement in MI segments from treated rats was related only to the absolute level of sestamibi uptake on pretherapeutic pinhole SPECT (P = 0.02; r = 0.39). No relationship was documented with the other parameters from pretherapeutic pinhole SPECT (end-diastolic volume, end-systolic volume, and ejection fraction).
99mTc-Sestamibi pinhole SPECT images of treated rat 1, 3, 5, and 7 mo after MI and, in center column, dual 111In/99mTc-sestamibi pinhole SPECT images 48 h after transplantation. BMSCs were injected in core of MI area with severe perfusion defects (<50% of sestamibi uptake), and no clear improvement in perfusion was seen after transplantation. A = anterior wall; I = inferior wall; L = lateral wall; and S = septal wall.
These results are illustrated in Figure 1B, where the MI segments have been categorized according to the presence, on pretherapeutic pinhole SPECT, of either severe perfusion defects (<60% uptake) or only moderate ones (60%–70% uptake). During the 3 mo after cell injection, the MI segments from treated rats had a much greater improvement in perfusion in the case of moderate perfusion defects (absolute changes in sestamibi uptake: +6% ± 6%) than in the case of severe ones (−1% ± 6%, P = 0.003) or in the 2 corresponding groups from untreated rats (−5% ± 3% for moderate defects and −0.4% ± 4% for severe defects, P < 0.001 for both). As evidenced in Figure 1C, furthermore, the perfusion improvement documented in segments with moderate perfusion defects before treatment was unaffected by the presence or absence of 111In-BMSCs 2 d after treatment.
DISCUSSION
Even when occurring belatedly, reperfusion of infarcted myocardium might improve patient outcomes (2). Recent studies have shown that the perfusion of infarcted areas could be increased without any revascularization procedure by transplanting potent angiogenic cells: adult BMSCs (4,7,8,20). When these cells are injected within infarcted myocardium, enhanced angiogenesis may be seen on histopathologic slices (7,8,21). This enhancement might relate directly to the injected BMSCs, which involve various endothelial precursors (3), or indirectly to stimulation of angiogenesis by cytokine production from BMSCs (22).
It is not known, however, whether myocardial perfusion is really enhanced at the exact sites of cell implantation and whether the enhancement depends on local metabolic factors, such as the residual levels of perfusion and viability. Better knowledge of these factors requires that the sites of cell implantation be characterized and followed up in vivo.
In humans, myocardial SPECT has been widely accepted as a gold standard for the sizing and localizing of infarcted areas (13,23), for determining LV function on gated SPECT acquisitions (14), and for assessing the improvement in perfusion relative to various treatments (24). With the use of pinhole collimators—and thanks to advances in data processing—we recently adapted myocardial SPECT to the investigation of adult rats (10). Furthermore, in other groups of rats with a surgically occluded LAD, we previously validated the use of this technique for identifying infarcted segments and following them, together with the parameters of LV function, by serial 99mTc-sestamibi pinhole gated SPECT (11) and for determining the distribution of transplanted BMSCs, according to the infarction site, by 111In-oxine labeling of BMSCs and dual-pinhole SPECT of activities from 99mTc-sestamibi and 111In (12). This 111In-labeling technique has already been used to track various implanted cells (24–26), and the usefulness of dual SPECT for separating 111In and 99mTc images has already been widely reported (28).
In the present study, rats with a surgically occluded LAD exhibited a varying number of underperfused MI segments on pretherapeutic 99mTc-sestamibi pinhole SPECT. This variability in MI areas was documented in our previous pinhole SPECT studies (11,12), as well as in several histopathologic studies performed on the same experimental model (1,29).
In our subgroup of treated rats, furthermore, the sites of early cell retention were far from closely matched to the targeted-MI ones. On dual-pinhole SPECT 48 h after treatment, the 111In-BMSCs could not be detected in 44% of the MI segments and were localized outside the MI segments, within adjacent segments, in nearly half the cases. This discrepancy was documented in our preliminary report (12) and may explained, first, by imperfections in the visual delineation of certain MI segments at the time of injection (subendocardial or septal MI); second, by difficulties with the cell-injection technique itself (small MI areas, rapid wall motion); and third, by the fact that only a single dose of BMSCs was injected within the MI core.
Moreover, subsequent follow-up has shown clear evidence of an improvement related to cell therapy. Perfusion of MI segments, as determined by quantified values of sestamibi uptake, was enhanced significantly in treated rats but worsened in untreated ones, and the rate of LV enlargement was slower in treated rats than in untreated rats. These observations are in accordance with previous studies showing that the angiogenic effects of BMSCs benefited LV remodeling (21,30).
However, improved perfusion in the MI segments of treated rats varied widely. Especially important was that this improvement did not predominate in segments showing 111In-BMSCs 2 d after treatment. As illustrated in Figures 3 and 4, this therapeutic effect seemed frequently to spread beyond the initial site of cell implantation, especially to adjacent segments. It is not known whether this observation relates to a subsequent migration of BMSCs (31) or to a regional diffusion of angiogenic paracrine factors, which are known to be secreted by BMSCs (30,32). Nevertheless, this observation gives evidence that the local variability in the therapeutic effect of BMSCs is not explained by the heterogeneous initial repartition of engrafted cells and that, therefore, other factors are involved.
These factors might be the metabolic conditions at and around the transplantation sites. In our treated rats, indeed, the sole but strong significant predictor of further improvement in perfusion was the level of sestamibi uptake on pretherapeutic pinhole SPECT. Cell therapy had a poor effect when segments had only a low level of sestamibi uptake before treatment. By contrast, in the MI segments with higher residual uptakes, such as those in the peripheral infarct zone, the therapeutic effect was marked, leading sometimes to the disappearance of any perfusion abnormality, as illustrated in Figure 3. This finding suggests, first, that the therapeutic effect of BMSC injection depends on the baseline blood supply at and around the injection sites. A sufficient baseline blood supply is, indeed, likely to be required for keeping the engrafted cells alive and, thereafter, for sustaining the active metabolic processes of angiogenesis.
Second, however, the MI segments with sufficient residual perfusion are also those with the higher proportion of viable myocardial tissue and with the lower density of fibrosis (33), and such tissue characteristics might influence the further development and differentiation of engrafted BMSCs. For instance, it was shown that these cells might preferentially differentiate into fibroblastlike cells when they are administered on wounded tissues (34) or cocultured with fibroblasts (35). One could therefore hypothesize that the therapeutic effect of injected BMSCs might not depend solely on the local blood supply but also on the cellular and metabolic characteristics of tissues lying at and around the implantation sites.
On the basis of the results from our previous study (25), we chose to inject a unique and limited number of BMSCs directly within the core of infarcted areas. This technique provided high percentages of cell retention and prevented the deleterious effects that might relate to a traumatic injection within normal myocardium (16). According to the results from the present study, however, a further improvement in perfusion is much higher in the MI segments showing a significant residual level of perfusion, such as those in the peripheral border zone. It may therefore be questioned whether our results could be enhanced with multiple injection sites covering the entire border zone and with the use of associated treatments that might enhance the perfusion of transplantation sites (antianginal drugs, revascularization). Such optimizations, leading to further improvements in myocardial perfusion, might also be associated with a more relevant functional improvement than the decrease in the rate of LV enlargement, which was documented in the present study.
These points will require further studies but already suggest that the characterization of optimal injection sites, as well as the choice of concomitant treatments, might be crucial for the success of cell therapy in individuals.
CONCLUSION
By applying an original pinhole SPECT technique in rats with chronic MI treated by intramyocardial injection of BMSCs, we could precisely characterize the initial sites of implanted cells. We also showed that the subsequent enhancement in perfusion predominated in infarcted areas that had a sufficient residual perfusion before treatment but not in infarcted areas at sites of initial cell engraftment. These findings give evidence of the dependency of the treatment on the perfusion and metabolic environment of implantation sites.
Acknowledgments
This study was supported by grants from the French Society of Cardiology (SFC, Paris, France), the Lorraine Association for Research and Transplantation (ALERT, Nancy, France), the Association for Research and Scientific Information in Cardiology (ARISC, Nancy, France), the Foundation of France (FDF, Paris, France), and the Foundation for Medical Research (FRM, Paris, France).
Footnotes
-
COPYRIGHT © 2007 by the Society of Nuclear Medicine, Inc.
References
- Received for publication October 5, 2006.
- Accepted for publication December 3, 2006.