Abstract
Even after recanalization of a chronic total coronary occlusion, functional recovery is incomplete and parts of the myocardium remain hypoperfused. In this randomized, placebo-controlled, and double-blinded study, we investigated relative changes in myocardial perfusion and glucose metabolism induced by intracoronary administration of blood-derived circulating progenitor cells (CPCs), compared with the natural course in a control group after recanalization of total coronary occlusion. Methods: After recanalization of total coronary occlusion, 26 patients were randomly assigned to the CPC treatment or placebo group. Regional myocardial perfusion and glucose metabolism were assessed by 99mTc-tetrofosmin SPECT and 18F-FDG PET at baseline (after recanalization of total coronary occlusion) and 3 mo after the administration of 69 ± 14 × 106 CPCs or cell-free serum, respectively. Segments were classified as “normal,” “perfusion–metabolism mismatch” (dysfunctional segments with a 99mTc-tetrofosmin–18F-FDG mismatch), or “scar.” Results: In contrast to the placebo group, CPC administration resulted in a significant decrease in the number of segments with a perfusion–metabolism mismatch, from 3.0 ± 0.5 to 1.7 ± 0.6 segments (P < 0.05 vs. baseline). Of the normal segments at baseline, 2.7% in the CPC group and 30% in the placebo group revealed a perfusion–metabolism mismatch at follow-up after 3 mo (P < 0.05 vs. placebo). Conclusion: Intracoronary administration of CPCs significantly reduces the amount of myocardium with a perfusion–metabolism mismatch and prevents areas with normal perfusion and metabolism after recanalization of total coronary occlusion from becoming dysfunctional during the next 3 mo. These results show that PET and SPECT can be used to monitor the effect of progenitor cells on myocardial integrity. More important, they provide evidence supporting expansion of the use of progenitor cell treatment to chronic coronary artery disease.
Patients with total occlusion of a main coronary artery are known to benefit significantly from recanalization with regard to major cardiac events (1), long-term survival (2), improvement of left ventricular function (3), and myocardial flow reserve (4). Nevertheless, functional recovery is incomplete, and parts of the myocardium remain with a perfusion–metabolism mismatch (5–7). However, the underlying mechanisms are not fully understood (7). One speculation is that parts of the myocardium are insufficiently supplied because of persistent paradoxical vasoconstriction of epicardial vessels and microvascular dysfunction (8).
Lately, experimental and clinical studies using progenitor cells have shown functional recovery and improved myocardial perfusion in chronic myocardial ischemia (9,10). To investigate whether progenitor cells also demonstrate such a benefit after recanalization of total coronary occlusion, we conducted a randomized, placebo-controlled, and double-blinded study with intracoronary administration of blood-derived circulating progenitor cells (CPCs) in patients after successful recanalization of total coronary occlusion. We recently published a paper focusing on the methodology of preparation and administration of the CPCs and inflammatory markers, invasive measurements of coronary endothelial function, and assessment of left ventricular function and wall motion with MRI (11). This previous publication also included an aspect of myocardial perfusion and metabolism data. In this present study, we focused on subtle analyses of relative changes in myocardial perfusion and glucose metabolism induced by CPCs, compared with the natural course in a control group, after successful recanalization of total coronary occlusion.
MATERIALS AND METHODS
The detailed methods of patient selection and of preparation and administration of the progenitor cells were described in our previous publication (11). Briefly, patients with total coronary occlusion, clinical signs or objective measures of myocardial and local wall motion abnormalities as assessed by left ventricular angiography were screened. Total coronary occlusion was defined as an occlusion of a native coronary artery for more than 30 d with no luminal continuity and with thrombolysis in myocardial infarction (TIMI) flow grade 0 or 1 (12). Recanalization with restoration of TIMI flow grade 3 and a residual stenosis of less than 30% was considered a technical success. After successful recanalization of total coronary occlusion, patients underwent 18F-FDG PET and gated 99mTc-tetrofosmin SPECT to measure myocardial glucose consumption and perfusion. Subsequently, all patients received filgrastim (300 μg of granulocyte colony-stimulating factor) by subcutaneous injections twice a day over a period of 4 d to increase the number of CPCs in the blood. To select for CPCs, mononuclear cells were purified out of 400 mL of venous blood collected from each patient and cultured ex vivo for 4 d in endothelial-specific medium (13,14). After successful recanalization of total coronary occlusion, 26 patients were randomly assigned to the CPC group (13 patients) or the placebo group (13 patients). At a mean of 10 ± 1 d after successful recanalization of total coronary occlusion, patients randomized into the CPC group received intracoronary administration of an average of 69 ± 14 × 106 CPCs, by repeated infusion into the vessel previously reopened, whereas patients randomized into the placebo group received cell-free serum. Ninety percent of the selected cells showed the typical features of CPCs, that is, binding to lectin and uptake of acetylated low-density lipoprotein. Neither the interventionalist nor the clinical investigators (including the nuclear medicine physician) were aware of the randomization results for the individual patients. 18F-FDG PET and gated 99mTc-tetrofosmin SPECT were performed 8 ± 1 d after successful recanalization (but before CPC or cell-free serum administration) and 3 mo after administration of CPCs or cell-free serum.
18F-FDG PET was performed using a full-ring ECAT EXACT HR+ PET scanner (Siemens/CTI). Nondiabetic patients received a single dose of 250 mg of acipimox (Olbemox; Pharmacia Upjohn) after an overnight fast. Sixty minutes later, 50 g of glucose were given orally. A mean dose of 370 MBq of 18F-FDG was injected intravenously, and PET images were acquired in 2-dimensional mode 1 h later during 30 min, including a 10-min transmission scan. Diabetic patients (n = 2 of the placebo group and n = 1 of the CPC group) underwent a standard hyperinsulinemic euglycemic clamp, and 18F-FDG was administered when glucose plasma levels started to decrease. The images were reconstructed with filtered backprojection using a Hann filter (full width at half maximum, 7.9 mm).
To acquire the perfusion scans, we injected 400 MBq of 99mTc-tetrofosmin while the patients were resting and after they had fasted overnight. Patients were encouraged to have a fatty meal (including 250 mL of milk) 15 min after injection, followed by 500 mL of water 45 min later. Image acquisition began 1 h after injection using a dual-head SPECT camera (Vertex camera; ADAC Laboratories) with detectors positioned at an angle of 90° and equipped with ultra-high-resolution collimators and two 153Gd line sources for attenuation measurement. Sixteen views per detector were recorded over a period of 80 s each, scanning from the 45° left posterior oblique to the 45° right anterior oblique positions. Images were recorded in gated mode with simultaneous electrocardiogram recording. The image data underwent standard reconstruction using an iterative algorithm with attenuation and scatter correction. Electrocardiogram-gated data were used to calculate regional wall motion, regional wall thickening, and functional parameters (QGS software; Cedars-Sinai Medical Center).
According to the recommended standardized nomenclature, the left ventricular myocardium was divided into 17 segments (15). The segments were assigned to the territories of the 3 major coronary arteries as recommended. Despite tremendous intersubject variability in the coronary artery blood supply to myocardial segments, there is consensus in the literature that it is appropriate to assign individual segments to specific coronary artery territories (16). The target region was classified as the region corresponding to the reopened chronic coronary occlusion (including segments in the border zone). Cerqueira et al. have provided a detailed description of segment assignment (15). The segment with the highest 99mTc-tetrofosmin uptake outside the target was designated the reference area. All other segments in the 99mTc-tetrofosmin and 18F-FDG studies were adjusted to this reference area by applying a computerized automatic procedure. In the 99mTc-tetrofosmin perfusion and 18F-FDG metabolism studies, relative radiotracer uptake in the segments was calculated at baseline and at follow-up after 3 mo.
The segments were classified into 3 categories based on the local 99mTc-tetrofosmin and 18F-FDG uptake. Segments with normal 99mTc-tetrofosmin uptake and normal 18F-FDG uptake (>80% of reference) were considered normal. Segments with a perfusion defect (normalized 99mTc-tetrofosmin uptake < 80%) were classified as perfusion–metabolism mismatch when a 99mTc-tetrofosmin–18F-FDG mismatch (increase in 18F-FDG uptake ≥ 10%, compared with 99mTc-tetrofosmin uptake) and a wall motion reduction (<50% of maximum on the electrocardiogram-gated images) were present. Segments with a perfusion defect were classified as scar tissue when a 99mTc-tetrofosmin–18F-FDG match was present (<10% difference in 99mTc-tetrofosmin and 18F-FDG uptake). Statistical analysis was performed using the SPSS software package, version 14.0 (SPSS Inc.). The Wilcoxon rank test was performed to detect significant differences between baseline and follow-up. The χ2 test was used to detect group differences. The significance level was set at a P value of less than 0.05.
RESULTS
A detailed report on the demographic, clinical, and angiographic data analysis; safety and side-effects of granulocyte colony-stimulating factor stimulation; and clinical follow-up, assessment of coronary endothelial function, and assessment of global left ventricular function parameters by MRI was given in our previous publication (11). Patients in the placebo group and patients receiving CPC did not significantly differ in demographic, clinical, and angiographic parameters or medication. Four patients (2 each from the placebo and CPC groups) did not undergo follow-up perfusion or metabolism imaging studies, because they withdrew their consent. Furthermore, 1 patient in the placebo group was excluded from the study because of reocclusion of the initially successfully reopened total coronary occlusion at the time of serum administration. In 1 patient in the placebo group, the 18F-FDG PET images could not be analyzed quantitatively because of a spillover of radioactivity from the intestine. Therefore, the complete follow-up could be performed only on 11 patients from the CPC group and 9 patients randomized to the placebo group. Initially, the CPC group comprised 90 segments in the target region and the placebo group 66. In the baseline studies, in the CPC and placebo groups 20 and 9 segments, respectively, were classified as scar; 33 and 24, respectively, as perfusion–metabolism mismatch; and 37 and 33, respectively, as normal.
Time-Dependent Changes in Segments Classified as Normal
At baseline investigation, that is, after recanalization but before the administration of CPCs or cell-free serum, patients in the CPC and placebo groups were found to have no differences in the mean number of normal segments within the target area. Compared with the baseline situation, at 3 mo the number of normal segments was significantly higher in the CPC group within the target area. This was not the case in the placebo group (Figs. 1 and 2; Table 1).
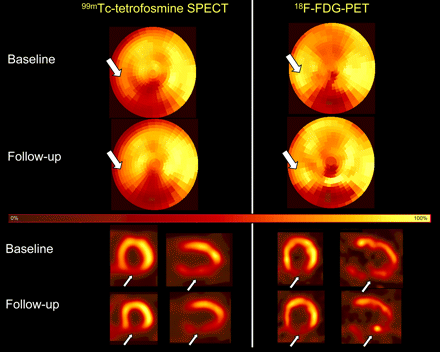
99mTc-Tetrofosmin SPECT and 18F-FDG PET studies (bull's-eye view and representative vertical long-axis and short-axis slices) of patient after recanalization of totally occluded left anterior descending coronary artery (baseline) and 3 mo after administration of CPCs (follow-up). Arrows indicate area with reduced 99mTc-tetrofosmin and enhanced 18F-FDG uptake corresponding to perfusion–metabolism mismatch in anterolateral area at baseline (arrows) and disappearance of this finding at follow-up.
99mTc-Tetrofosmin SPECT and 18F-FDG PET studies (bull's-eye view and representative vertical long-axis and short-axis slices) of patient after recanalization of totally occluded right coronary artery (baseline) and 3 mo after administration of cell-free serum (follow-up). Arrows indicate area with reduced 99mTc-tetrofosmin and enhanced 18F-FDG uptake corresponding to perfusion–metabolism mismatch in posteroseptal area at baseline. In this area, slight deterioration in perfusion occurs at follow-up.
Mean Number of Segments Within Target Area
Follow-up revealed that 94.6% of the segments classified as normal on the baseline scans remained normal within the target area in the CPC group (2.7% turned into scar and 2.7% developed a perfusion–metabolism mismatch), whereas in the placebo group 69.7% stayed normal and 30.3% revealed a perfusion–metabolism mismatch (Fig. 3A). These group differences in the fate at follow-up of segments that had been normal on the baseline scans were significant (P = 0.004, χ2 test).
Three-month follow-up of segments classified as normal (A) or having perfusion–metabolism (p/m) mismatch (B) within area of total coronary occlusion (target area) at 8 ± 1 d after recanalization. For both situations, group differences were significant (P < 0.05, χ2 test).
Time-Dependent Changes in Segments with a Perfusion–Metabolism Mismatch
Initial results on the fate of segments with a perfusion–metabolism mismatch on the baseline scans were presented in our previous publication (11). At baseline, the mean number of segments with a perfusion–metabolism mismatch within the target area did not differ between patients in the CPC and placebo groups. Compared with the baseline situation, the number of segments with a perfusion–metabolism mismatch at 3 mo was significantly lower in the CPC group within the target area. This was not the case in the placebo group (Table 1).
Follow-up revealed that 48.5% of the segments displaying a perfusion–metabolism mismatch on the baseline scans became normal within the target area in the CPC group (9.1% turned into scar and 42.4% remained with a perfusion–metabolism mismatch), whereas in the placebo group 12.5% became normal, 66.7% remained with a perfusion–metabolism mismatch, and 20.8% turned into scar (Figure 3B). These group differences in the fate at follow-up of segments that had shown a perfusion–metabolism mismatch on the baseline scans were significant (P = 0.004, χ2 test).
Time-Dependent Changes in Segments Classified as Scar
The mean number of regions classified as scar did not change significantly in either the CPC or the placebo group (Table 1).
Changes in 99mTc-Tetrofosmin Uptake
The relative 99mTc-tetrofosmin uptake increased slightly but significantly from 75% ± 15% (mean ± SD) to 78% ± 16% in the 90 segments within the target area after the infusion of CPCs (P = 0.02, Wilcoxon rank test). In segments of the placebo group, no relative change in 99mTc-tetrofosmin uptake was observed (74% ± 18% at baseline and 74% ± 17% after 3 mo). Of the 55 segments with reduced 99mTc-tetrofosmin uptake at baseline, 33% (18 segments) showed normal 99mTc-tetrofosmin uptake at follow-up in the CPC group. In the placebo group, of the 32 segments with reduced 99mTc-tetrofosmin uptake, 12% (4 segments) demonstrated a normal 99mTc-tetrofosmin uptake at follow-up. These group differences in the relative 99mTc-tetrofosmin uptake were significant (P = 0.036, χ2 test). However, because of the limited number of patients and the interindividual variations, an analysis of these changes on a per-patient basis did not reach the significance level.
The changes in the number of segments classified as normal, perfusion–metabolism mismatch, and scarred between baseline and follow-up within the target area are plotted individually on a per-patient base for the CPC and placebo groups in Figure 4. Normal segments increased and segments with perfusion–metabolism mismatch decreased in most CPC patients.
Change in number of segments classified as scar, perfusion–metabolism (p/m) mismatch, or normal in each patient from baseline to follow-up, for CPC group (A) and placebo group (B). Each patient is represented by 1 line. In most CPC patients, normal segments increase and segments with perfusion–metabolism mismatch decrease. Table 1 provides the statistics.
DISCUSSION
Our study demonstrated that, compared with the natural course after recanalization of total coronary occlusion, administration of CPCs after recanalization brought about significant differences in perfusion and glucose metabolism in the myocardial target area.
Even after successful recanalization of total coronary occlusion, parts of the myocardium remain nonfunctional, with a hypoperfusion–metabolism mismatch (6). This was the case in 36% of the segments in the target area in our study at 8 ± 1 d after recanalization, whereas 45% showed normal perfusion at that time. In the time until the 3-mo follow-up, 30% of normal segments in the recanalized area developed a perfusion–metabolism mismatch in the group that had not been given progenitor cells. This deterioration could be attributable to disturbances in microvascular circulation or insufficient blood flow due to persistent paradoxical vasoconstriction of epicardial vessels (8). Because only patients with a successful recanalization (TIMI flow grade 3 with a residual stenosis of <30%) were included in the study and no flow-limiting in-stent restenosis occurred (11), the deterioration in perfusion and development of a perfusion–metabolism mismatch in the placebo group was likely due to disturbances in microvascular circulation. We would hypothesize that the observed changes in perfusion rather reflect the natural time course after reopening of total coronary occlusion, with a short-term improvement (day 8) and subsequent deterioration (3 mo) due to disturbances in microcirculation in the placebo group. To our knowledge, no studies have investigated changes in perfusion and metabolism in a comparable interval shortly after recanalization of total coronary occlusion. Bax et al. performed follow-up studies 3 and 14 mo after surgical revascularization (17) and demonstrated a reduction of dysfunctional segments, with perfusion–metabolism mismatch in the later course. However, the situation immediately after recanalization is unknown, and the percentage of scarred tissue 3 mo after revascularization in their study was significantly higher than in our patients (55% vs. 10%, respectively).
Intracoronary infusion of CPCs after successful recanalization of total coronary occlusion not only led to a recovery of relative myocardial perfusion over 3 mo and a normalization of myocardial glucose metabolism in a part of the target area but also prevented myocardium with normal perfusion and glucose metabolism early after recanalization of total coronary occlusion from developing a perfusion–metabolism mismatch during the next 3 mo. Normalization of glucose metabolism and myocardial perfusion was seen in nearly 50% of the segments with an initial perfusion–metabolism mismatch within the target area. A significantly higher percentage of segments initially classified as normal stayed normal in the CPC than in the placebo group.
Various studies have suggested an improvement in clinical symptoms and myocardial stress perfusion by progenitor cell transplantation in patients with chronic ischemic heart disease (10,18–20). In these studies, progenitor cells were administered without a revascularization procedure and mainly by transendocardial injection. An animal study assessing the effect of the sole administration of bone marrow–derived progenitor cells after chronic myocardial infarction without recanalization procedures detected a greater improvement in myocardial perfusion or even normalization of perfusion by 99mTc-sestamibi pinhole SPECT if perfusion defects were less severe in the initial stage (21). Improvement was maximal in segments with a significant residual level of perfusion, such as those in the border zone of myocardial infarction. Because this area is where a mismatch in perfusion and metabolism mainly occurs, this finding does indeed argue for beneficial effects of progenitor cells in the state of single-vessel total coronary occlusion, where perfusion is at least partially obtained—i.e., because of collateral vessels (22). The effect of progenitor cells could be due to normalization of endothelial function and the subsequently improved microvascular and macrovascular function. Additionally, an advancement of endothelium-dependent vasodilatation due to the reconstitution of damaged endothelium by CPCs (23,24) or the release of survival factors by CPCs and thus the promotion of survival and proliferation of resident endothelial cells within the epicardial coronary arteries by those cytokines (25) has been supposed.
Our study found no significant changes in scarred segments independent of the administration of progenitor cells. This finding tallies with other progenitor cell therapy studies of chronic ischemia. In a small study including 7 patients, Tossios et al. (26) noted no significant improvement in myocardial viability after the concurrent transplantation of bone marrow–derived progenitor cells in patients undergoing coronary artery bypass surgery. In the study by Beeres et al. (10), the percentage of myocardial segments with some extent of scarring remained unchanged at the 3-mo follow-up. However, some investigators found a significant increase in myocardial viability in nonacute infarction 3 mo after surgery and progenitor cell administration (27).
One limitation of our study is the lack of perfusion and metabolism data before the recanalization of total coronary occlusion. However, because a considerable percentage of recanalization fails in total coronary occlusion, successful recanalization of total coronary occlusion was a prerequisite for randomization into the placebo or CPC group. It would be of interest to analyze the course of perfusion and metabolism changes from the initial situation of chronic ischemia before recanalization.
Our data might in principle be compromised by the fact that myocardial perfusion was studied by a SPECT tracer whereas glucose metabolism was measured by PET. However, because of the correction for measurement attenuation in the SPECT study, the influence of attenuation effects ought to be minimized (28), thus providing valid data for a segmental analysis.
Despite randomization, localization of the total coronary occlusion differed significantly between the CPC and placebo groups. The possibility that this difference affected our results cannot be ruled out.
CONCLUSION
Intracoronary administration of CPCs after successful recanalization of total coronary occlusion leads to a treatment-specific normalization of myocardium with a perfusion–metabolism mismatch resulting in a reduction of myocardium at risk. In addition, CPCs seem to have a positive effect on myocardial areas with normal perfusion and metabolism shortly after recanalization of total coronary occlusion, thus preventing the redevelopment of a perfusion–metabolism mismatch during the next few months. This prolonged effect suggests a slowing of disease progression due to CPCs. Further longitudinal studies should address whether both effects reduce the risk of cardiac events over longer periods.
Footnotes
-
COPYRIGHT © 2008 by the Society of Nuclear Medicine, Inc.
References
- Received for publication August 24, 2007.
- Accepted for publication December 20, 2007.