Abstract
PET is a sensitive technique for the identification of viable myocardial tissue in patients with coronary disease. Metabolic assessment with 18F-FDG is considered the gold standard for assessment of viability before surgical revascularization. Prior research has suggested that viability may be assessed with washout of 82Rb between early and late resting images. Our objective was to determine whether assessment of myocardial viability with 82Rb washout is reliable when compared with PET using 18F-FDG. Methods: We performed PET for 194 patients referred for PET 18F-FDG/82Rb to assess viability for clinical indications. We included 151 patients with resting defects >10% of the left ventricle (LV) (n = 159 defects). Patients with smaller resting 82Rb defects (<10% LV) were excluded for the purpose of this study. PET images acquired with 82Rb and 18F-FDG defined viability by the mismatch between metabolism and perfusion (18F-FDG >125% of 82Rb uptake in the 82Rb defect). Evidence of viability with 82Rb was assessed by the presence of (i) severity: 82Rb counts in the defect >50% of 82Rb in the normal zone of the resting PET images; (ii) washout: decrease of 82Rb counts in the defect from early to late resting 82Rb images <17% between the first 90-s image and the final 300-s image; or (iii) combined severity and washout criteria, which required positive criteria for (i) and (ii) to indicate viability. Results: Prevalence of viability by 18F-FDG/82Rb criteria was 50% (n = 79). Severity criteria yielded a sensitivity of 76% and a specificity of 17%, washout criteria yielded a sensitivity of 81% and a specificity of 23%, and both criteria had a sensitivity of 63% and a specificity of 32%. Positive and negative predictive values were poor for all criteria. No correlation existed between 82Rb washout and 18F-FDG–82Rb mismatch (r2 = 0.00). Multiple receiver-operating-characteristic plots showed very poor discrimination despite varying criteria for viability by 82Rb (severity from 50% to 60% of normal zone, washout from 12% to 17%). Conclusion: 82Rb washout from early to late resting images, combined with quantitative severity of the resting 82Rb defect, did not yield results equivalent to PET 18F-FDG–82Rb mismatch and may not accurately assess myocardial viability.
PET has been used as a sensitive technique to assess myocardial tissue viability in patients with coronary artery disease (1–6). After a myocardial infarction, overlying surviving tissue may be ischemic (inadequate increment in blood flow during stress), stunned (contractile function temporarily depressed despite restored perfusion after a period of ischemia), or hibernating (contractile function temporarily depressed, possibly an adaptive mechanism against ischemia, which might result from impaired perfusion at rest) (6–9). Preserved or absent viability as assessed with 18F-FDG PET has become an important technique because of its ability to predict functional outcome after revascularization of ischemic or hibernating myocardium (7–9). Moreover, a flow–metabolism mismatch on 18F-FDG PET has been associated with an elevated risk of subsequent cardiac events in patients treated without revascularization (10–12).
PET with 82Rb has been validated for the detection and functional assessment of coronary artery stenoses and infarct size (13–20). Previous research has indicated that 82Rb is taken up briefly but clears rapidly from irreversibly injured tissue, but 82Rb is retained in reversibly injured but viable myocardium (21,22). Clinical studies followed that compared early and late tracer activity to estimate the rapidity of washout as a method to establish viability. This could result in potential cost savings by avoiding the need for cyclotron-produced 18F-FDG and could avoid close glucose control during 18F-FDG administration (23,24).
Our purpose was to evaluate the applicability of 82Rb washout to an unselected clinical population. We defined myocardial viability for this study as the mismatch between 18F-FDG and 82Rb (where 18F-FDG was >125% of 82Rb) and compared this measurement with each of the following 3 82Rb criteria: (i) severity: 82Rb counts in the defect >50% of 82Rb in the normal zone of the resting PET images; (ii) washout: decrease of 82Rb counts in the defect from early to late resting 82Rb images <17% between the first 90-s image and the final 300-s image; or (iii) combined severity and washout criteria, which required positive criteria for (i) and (ii) to indicate viability.
MATERIALS AND METHODS
Patient Population
Consecutive patients were referred to Emory Crawford Long Hospital for assessment of viability from August 1993 through June 1996 and from February 2001 through October 2001 and were evaluated for retrospective analysis of their data. We began the analysis with the early (1993–1996) group, but added patients at a later date (2001) to compare results. Protocols and results were the same for these 2 time periods. On the basis of clinical criteria, patients did not undergo 18F-FDG testing if they were found to have a resting defect <5% of the left ventricle (LV) on initial 82Rb imaging and were excluded from the study. After the 18F-FDG study, patients with 82Rb defects <10% of the LV were also excluded because these defects were thought to have limited clinical prognostic significance and the potential for confusion due to proximity of the defect to the adjacent normal tissue. A total of 194 patients were referred for cardiac PET viability assessment. We identified 151 patients with 159 late resting 82Rb defects involving ≥10% of the LV mass (Table 1). Study design and protocols for retrospective data analysis were reviewed and approved by the Institutional Review Board.
Study Patient Characteristics
Myocardial Imaging Protocol
PET was performed using a Posicam camera (Positron Corp.) producing a 21-slice tomogram with a 125-mm field of view, a spatial resolution of 5–6 mm in the x–y plane, 10–12 mm in the z-axis, with a high sensitivity and counting rate performance. Standard PET image equipment and techniques were used as previously described (25,26).
Because patients were scheduled for myocardial stress imaging with dipyridamole along with viability assessment, patients were instructed to fast for at least 8 h and to avoid caffeine and theophylline before the test. The patient had an initial pilot or “scout” scan with infusion of 740 MBq 82Rb after continuous elution from the 82mSr generator. This pilot scan image was used to ensure that the patient’s LV was within the 12.5-cm field of view of the camera and to estimate the time required to clear 82Rb from the LV cavity blood pool. In most patients, we waited approximately 80 s after completing the 1-min infusion of 82Rb before beginning acquisition of PET images. In patients with larger LV cavities and slower rates of clearance of 82Rb, we increased the delay time between 82Rb infusion and starting image acquisitions from 80 to as much as 120 s, to allow clearance of 82Rb from the cardiac blood pool. We acquired 2 resting images after infusing 1,480–2,220 MBq 82Rb: the first image (early) for 90 s and the second image (late) for 300 s, beginning as soon as the first image was acquired. Next, we acquired the transmission scan to be used for attenuation correction, beginning 10 min after the first 82Rb infusion and ending just as infusion of dipyridamole was completed (0.142 mg/kg/min for 5 min intravenously). Next, we began infusing 82Rb, 1,480–2,220 MBq intravenously, to match the dose given at rest. We acquired the “stress” 82Rb scan, beginning 6 min after beginning the dipyridamole infusion or after there had been a 10% increase in heart rate or a 10% decrease in blood pressure. When obtained, stress images were not used in the determination of viability. Most patients received aminophylline (75–125 mg intravenously) to reverse side effects of dipyridamole.
Patient preparation for the 18F-FDG imaging included measurements of glucose by a finger prick, before starting the 82Rb study. As previously described (25), and based on the initial fasting glucose level, we administered glucose, as 25 g/50 mL (mixed with hydrocortisone [5 mg] to minimize local irritation of the vein). We repeated the serum glucose measurement 30–60 min after the glucose load and administered either regular intravenous insulin or more glucose, as dictated by the glucose level. If necessary, the measurement of glucose and administration of insulin were repeated, to achieve a stable glucose level before administering the 18F-FDG (370 MBq intravenously). We began imaging the heart 60 min after the 18F-FDG was injected.
Image Analysis
The attenuation-corrected tomographic transaxial images, 5-mm thick, were reoriented to vertical long-axis, horizontal long-axis, and short-axis views using the Positron software package. The short-axis views were summarized into polar maps using clinically validated in-house software. Each polar map was quantitated in terms of counts per voxel in each of 1,200 voxels in the LV.
A 82Rb normal file of 50 subjects (25 women, 25 men) with <5% probability of coronary artery disease was previously created (26). A normal zone of each patient’s LV was defined as the voxels with counts greater than minus 1 SD from the normal file. Each voxel was also defined by its SDs below normal. Count distributions between different regions were compared by quantitation of LV voxels that fell below the mean of the normal file by threshold values of 2.5 SDs. We used a computer program to copy the defect and normal zones on resting 82Rb to the 18F-FDG polar map. These defects were then compared for the difference between count ratios for the defect zone/normal zone for 82Rb and 18F-FDG in the same defect.
The counts in the defect regions (PDZ = patient’s defect zone) were expressed as a percentage of the counts in the normal region (PNZ = patient’s normal zone) (Table 2). The severity of the defect was determined by comparison of the average counts per voxel in the defect zone compared with the average counts per voxel in the normal zone in the late resting 82Rb perfusion scan. The late images were used to minimize the influence of background from the LV cavity blood that appears to diminish to a stable low level within 3–4 min after injection (24):
 The normal region was identified as all pixels with counts greater than minus 1.0 SD from the mean of the normal file, in that region. The “82Rb washout” was calculated as the change in the average count ratios ([PDZ]/[PNZ]) in each region within the defect from early and late images and normalized to the early images using the following formula:
The normal region was identified as all pixels with counts greater than minus 1.0 SD from the mean of the normal file, in that region. The “82Rb washout” was calculated as the change in the average count ratios ([PDZ]/[PNZ]) in each region within the defect from early and late images and normalized to the early images using the following formula:
 This percentage was used as a quantitative assessment of the degree of rubidium “washout” in each region of the resting scan, where a higher percentage represents a faster washout and less evidence of viability. The late resting image SD polar map, mapped to the early resting images, again defined defect and normal zones.
This percentage was used as a quantitative assessment of the degree of rubidium “washout” in each region of the resting scan, where a higher percentage represents a faster washout and less evidence of viability. The late resting image SD polar map, mapped to the early resting images, again defined defect and normal zones.
Definitions of Terms Used in Text
The late rest 82Rb defect and normal zones were mapped to the 18F-FDG polar map, and the ratio of average counts per voxel in the PDZ to average counts per voxel in the PNZ was measured. The ratios were compared to determine whether the defects were matched (18F-FDG ≤ 82Rb) or mismatched (18F-FDG > 82Rb). Viability was assessed by the uptake of 18F-FDG within the region of the defect observed on the late 82Rb scan: the relative counts (PDZ/PNZ) in each region within the defect from 18F-FDG and late 82Rb images were determined and compared using the following formula and termed “mismatch”:
 Mismatch percentage was used as a quantitative assessment of the degree of 18F-FDG–82Rb mismatch in each region, where a higher percentage represents a greater amount of viable myocardium overlying the defect.
Mismatch percentage was used as a quantitative assessment of the degree of 18F-FDG–82Rb mismatch in each region, where a higher percentage represents a greater amount of viable myocardium overlying the defect.
To evaluate the effect of background counts, all patients were qualitatively evaluated for LV blood-pool activity on early and late 82Rb images and classified as having a mild, moderate, or severe change (decrease) in LV cavity counts from early to late resting 82Rb images. Those with severe change were considered to have a large background from 82Rb in the LV cavity on early images. In 3 patients from each subgroup, regions of interest (ROIs) were identified on short-axis images, in the following locations: LV cavity, maximum counts in LV myocardium, and minimum counts in LV myocardium. Counts were measured in each region on early and late resting images, and washout was recalculated, correcting for early (CE) and late (CL) background counts:

Statistical Analysis
All data analysis was performed using an SPSS statistical software program. Descriptive and frequency information was evaluated by mean, SD, maximum and minimum, and confidence intervals. Distributions were displayed visually by frequency histograms. Standard scatter-plot analysis was performed using linear regression analysis and 95% confidence intervals. Sensitivity and specificity was calculated by standard 2 by 2 table evaluation of viability, where the 18F-FDG–82Rb mismatch was considered, for this purpose, to define the presence of viable myocardium. We also varied 82Rb criteria (severity threshold, 0.40–0.65; washout threshold, 12.5%–25%; and combined severity and washout) and 18F-FDG criteria (mismatch >125%, >135%, >145%). A 2-tailed P value of <0.05 was used for statistical significance. Continuous variables are presented as mean ± SD and categoric variables are presented as percentages. To assess whether observed differences were significant, we used χ2 tests, the Student t test, and ANOVA, requiring a 2-tailed P < 0.05 to be considered significant.
RESULTS
One hundred fifty-one patients with 159 defects involving ≥10% of resting LV myocardium were studied (Table 1). There were 74% men, and the average age was 62.2 y. PET 18F-FDG–82Rb mismatch indicated viability in 79 defects (50%), with a mean resting defect size of 29.4% of LV (range, 10%–57%). The mean severity of the resting defects was 0.56 (range, 0.39–0.73), with a mean washout of 12% (range, −18% to 30%).
The severity of 82Rb defect (PDZ/PNZ) showed poor correlation with viability, as measured by 18F-FDG–82Rb mismatch (r2 = 0.01), and washout of 82Rb showed poor correlation with viability by 18F-FDG–82Rb mismatch (r2 = 0.00) (Fig. 1). We also tested whether 82Rb criteria might provide the ability to discriminate viable versus nonviable myocardium, despite limited quantitative correlations with 18F-FDG mismatch. The sensitivity (81%) of 82Rb washout to predict viability by 18F-FDG–82Rb mismatch was reasonable, but the specificity was poor (23%). The positive predictive value (51%) and negative predictive value (56%) were limited. Changing the threshold criteria for 82Rb washout (Fig. 2) did not improve accuracy. We also computed correlations for subgroups of patients to test other factors that might have influenced the study: Patients who had dipyridamole testing (n = 133) showed the same poor correlations between 82Rb washout and 18F-FDG–82Rb mismatch (r2 = 0.00) as did patients without dipyridamole testing (n = 18) (r2 = 0.00). Also, patients with small- to moderate-sized (10%–29% LV) defects showed similar poor correlations between these 2 variables (r2 = 0.00) as did patients with large (>29% LV) defects (r2 = 0.00).
Scatterplot shows poor correlation between percentage washout of 82Rb between early and late resting images and percentage mismatch between metabolism (18F-FDG) and perfusion (82Rb uptake) in 82Rb defect used for assessment of viability.
Bar graph displays sensitivity (filled bars) and specificity (open bars) of 82Rb defect severity at various cut points when compared with viability using 18F-FDG–82Rb mismatch.
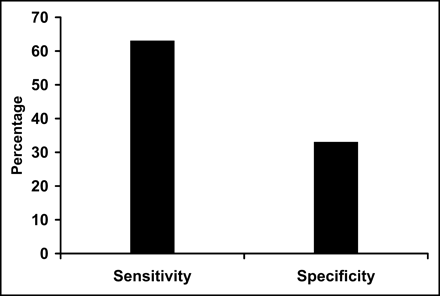
Similarly, the sensitivity (76%) of 82Rb defect severity to predict 18F-FDG mismatch was reasonable, but poor specificity (17%) limited diagnostic utility (Fig. 3). The positive predictive value (48%) and negative predictive value (42%) were limited. Finally, when both defect severity and 82Rb washout were combined, the sensitivity (63%), specificity (32%), positive predictive value (48%), and negative predictive value (47%) indicated limited usefulness (Fig. 4).
Bar graph displays sensitivity (filled bars) and specificity (open bars) of 82Rb defect washout at various cut points when compared with viability using 18F-FDG–82Rb mismatch.
Sensitivity and specificity of combined 82Rb severity and mismatch used to define viability when compared with viability assessment using 18F-FDG–82Rb mismatch.
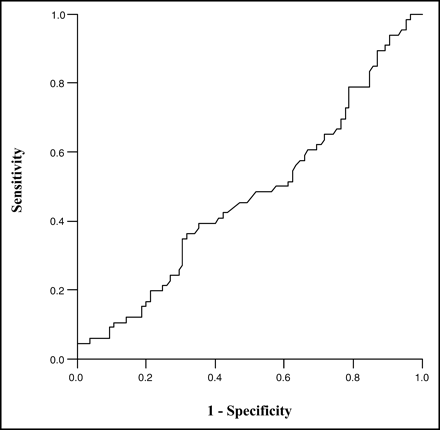
Several criteria for viability were evaluated by constructing receiver-operator-characteristic (ROC) curves of false-positive rates (horizontal axis) versus true-positive rates (vertical axis) (Fig. 5). Sensitivity (true-positive rate) and specificity (1 − false-positive rate) were plotted over a wide range of criteria for 82Rb viability (severity, >0.40–0.65; washout, <12.5%–25%), but they provided little ability to discriminate whether a defect would show myocardial viability by 18F-FDG–82Rb mismatch criteria.
ROC curve compares 82Rb washout with 18F-FDG–82Rb.
We analyzed a subgroup of 9 patients to assess the impact of background subtraction: 3 from each group having a qualitatively mild, moderate, or severe change in LV cavity counts—that is, background, from early to late resting images. Results suggested that background count subtraction did not change the 82Rb washout results and did not improve the ability of 82Rb defect severity or washout to predict the 18F-FDG–82Rb mismatch. Also, when patients were grouped by background activity, the group with little or no change in background activity between early and late images did not show better correlation between 82Rb washout and 18F-FDG–82Rb mismatch. Finally, after the exclusion of all diabetic patients from the analysis, correlation was not improved (r2 = 0.00).
Figures 6 and 7 show early 82Rb, late 82Rb, and 18F-FDG displays from 2 patients with discrepant 82Rb washout and 18F-FDG viability results. Short-axis slices in the top row (Fig. 6) show a small, mild anterior septal defect on early 82Rb (left image) and a larger, more severe anterior septal defect on late 82Rb (middle image)—with the dramatic washout from early to late suggesting nonviable myocardium. One complication is illustrated in this patient: the high level of background 82Rb in the LV cavity on the early 82Rb image (left) that decreases dramatically on the late 82Rb image (middle). Even this level of background does not seem capable of explaining the magnitude of washout from the myocardial defect. The 18F-FDG images show only a mild defect (right) to indicate a marked mismatch between the severity of defects on 18F-FDG (mild) and late 82Rb (severe) that indicates viable myocardium. The bottom row shows horizontal long-axis images of one patient during resting acquisitions from left to right: during the early 82Rb acquisition, the late 82Rb, and during the 18F-FDG acquisition. The early 82Rb image (left) shows an apical defect that looks quite similar in severity and size on the late 82Rb image (middle) to suggest viable myocardium, because the 82Rb present early did not wash out very much on late 82Rb images. The 18F-FDG images (right) show a defect that matches the late 82Rb images to indicate nonviable myocardium, because the perfusion (late 82Rb) and metabolic (18F-FDG) scans show closely matched severity and size of defects. These findings are confirmed in the bull’s-eye views (Fig. 7).
Patient 1 (short-axis slices): nonviable by 82Rb washout, viable by 18F-FDG–82Rb. Patient 2 (horizontal long-axis slices): viable by 82Rb washout, nonviable by 18F-FDG–82Rb.
Bull’s-eye summary views of patients 1 and 2 shown in Figure 6.
DISCUSSION
Previous studies (21,22) evaluated the retention kinetics of rubidium as a reflection of cell membrane integrity, using a β-detector mounted in a needle and inserted into the myocardium of dogs to measure local β-emissions from 82Rb. On the basis of this work, others (23,24,27) proposed using 82Rb instead of 18F-FDG–82Rb mismatch to assess myocardial viability, suggesting that loss of cell membrane integrity, demonstrated by rapid clearance of 82Rb, would parallel the loss of metabolic activity as measured with 18F-FDG. These studies raised the possibility that substantial savings might be possible by 82Rb viability assessment because of the elimination of cyclotron-produced 18F-FDG.
The present study found that PET 82Rb imaging alone could not identify myocardial viability as defined by PET 18F-FDG–82Rb mismatch results. We found no correlation between viability results obtained with 82Rb washout or defect severity and the mismatch between the metabolic tracer 18F-FDG and the flow tracer 82Rb. When, for the purpose of this study, we defined viability as the mismatch between 18F-FDG and 82Rb, the sensitivity, specificity, positive predictive value, and negative predictive value were poor for either resting 82Rb defect severity or washout analysis.
Why did our results differ from these earlier results? Possible reasons include problems with the acquisition of early and late 82Rb images, inaccurate evaluation of the 82Rb washout and severity, and problems with the 18F-FDG results to which 82Rb data were compared. However, the methods used in the current study are in daily use in our laboratory, which has now studied >16,000 patients with 82Rb and >900 patients with 18F-FDG for myocardial viability. The protocols are standardized and consistent with recent guidelines (25).
In terms of the 82Rb washout analysis, one critical factor is the time between infusion of 82Rb at rest and beginning the acquisition of the early resting PET images. It is crucial to wait for 82Rb to clear from the blood, because 82Rb counts in the cavity can also scatter into subendocardial defects, making the resting defect appear artificially mild on rest 82Rb, which would suggest ischemia (compared with stress 82Rb) or viability (based on the mildness of the resting defect), or absence of viability (based on marked washout from early myocardial images contaminated by cavitary 82Rb). The time required for clearing 82Rb from the cardiac blood pool is related inversely to the cardiac output and directly to the circulation time. After dipyridamole, the cardiac output increases and the circulation time decreases, so that 82Rb is cleared from the cardiac blood pool more quickly. If background counts could be subtracted accurately, 82Rb blood-pool clearance would not be an issue. Background subtraction, however, is difficult in PET for several reasons. The spatial resolution, measured at full width half maximum, is similar to or larger than the LV wall thickness, rather than being half the value of the object being measured (28). Further, drawing a ROI is difficult and the LV cavity is not the only source of background—for example, the background for the subendocardial defects should emphasize LV cavitary 82Rb as background, whereas the normal tissue in the subepicardium should emphasize the different extracardiac sources of background. Even the location of the ROI for background within the LV cavity deserves attention. The results of the analysis of a subgroup of patients suggested that correction for background counts did not change the study results. Further, automated background subtraction, needed for reproducibility and objectivity, does not exist on current commercially used systems.
82Rb defect severity corresponded somewhat more closely with the 18F-FDG–82Rb mismatch than did the washout analysis, but the agreement was still poor. This lack of agreement with severity analysis is harder to explain than the problem with the more complicated washout analysis. A large body of data from animal studies with microsphere measurements after myocardial infarction shows that reduction in average transmural blood flow on a resting study after coronary occlusion correlates with the extent of infarction. This extent of infarction was measured as depletion of myocardial creatine kinase and histologic damage, especially its extension from endocardium to epicardium (21,22). Differences between microsphere data and our PET data suggest that the imaging characteristics of the PET camera are the chief factors limiting the ability of this approach to measure viability (29), but the extraction fraction of 82Rb, which is somewhat higher in regions with slower flow, may also contribute to this problem (30).
In the present analysis, we defined the defects with greater resolution and reproducibility than in the other studies cited by using a polar (“bull’s-eye”) map of the defect. This map used an automated computer program with clearly defined criteria to determine the percentage of the LV voxels that were abnormal, based on their having counts reduced by 2.5 or more SDs below the average of the normal file. Thus, it was not necessary to introduce new sources of error when using either ROIs defined by an observer or arbitrarily drawn segments that are less likely to correspond to anatomic vascular distribution. Analysis of arbitrarily assigned segments, as done in prior studies, inevitably leads to sampling a mixture of normal and abnormal myocardium within most segments. The identification of perfusion defects by objective criteria written into a computer program allows unambiguous, reproducible definition of an abnormal region (25).
For a technique to gain widespread acceptance, it should be easy to use and widely applicable to a variety of clinical environments and populations. Our study suggests that 82Rb washout used in a “real-world” setting yields results that differ considerably from 18F-FDG–82Rb mismatch. It is possible that the larger and unselected patient population in our study provided a broader basis for comparison with the general population. Our only patient selection criterion was referral by a cardiologist or cardiac surgeon for viability assessment and that they showed a resting perfusion defect that reduced counts by >2.5 SDs, and occupied >10% LV mass, so that milder defects (22% of patients initially referred) were excluded. It is possible that other studies have included some patients with milder, smaller defects that would not be most relevant to clinical decisions regarding revascularization and viability.
Alternatively, if drawing the defect ROI led to smaller defects with uniform severity by avoiding the edge of the defect, then the defects might have appeared somewhat smaller but more severe. We examined these effects by comparing automatically defined defects that were small or moderate (≤29% LV, n = 81) versus large (>29% LV, n = 78), based on a median defect size of 29% LV. There was no difference in the correlation between 82Rb washout and 18F-FDG–82Rb mismatch based on small or moderate defect size (r2 = 0.00) versus large defect size (r2 = 0.00), suggesting that the conclusion of the present study was not determined by potential artifacts arising from the partial-volume effect and differences in defect size (28). The performance of 82Rb stress myocardial perfusion imaging is also a consideration. Although it is recognized that regional 18F-FDG uptake may be affected by prolonged metabolic effects of any stress-induced ischemia, stress-induced ischemia during persantine stress is uncommon and, therefore, unlikely to alter the overall findings of this study.
The lack of correlation between the numeric indices of viability obtained with 82Rb washout and 18F-FDG–82Rb mismatch does not necessarily mean that resting 82Rb cannot predict other useful clinical information. It is troubling for clinical applications, however, that 82Rb severity or washout criteria were not able to discriminate the presence or absence of viable myocardium, as described by their sensitivities and specificities, compared with the 18F-FDG–82Rb mismatch. Because the 2 techniques do not produce similar viability assessments, more study is necessary to establish which method is better able to predict subsequent events, such as clinical outcomes or changes in cardiac function after revascularization. PET with perfusion and metabolism of 18F-FDG has been validated to correlate with return of myocardial function after revascularization.
CONCLUSION
These results show that resting 82Rb imaging results, analyzed for defect severity or washout, differ from the assessment offered by 18F-FDG–82Rb mismatch. This study suggests caution in using 82Rb alone as a measure of myocardial viability. Further study of 82Rb is needed to assess the ability of 82Rb to predict outcomes after revascularization.
Acknowledgments
The authors thank our patients, the PET center nursing and technologist staff, the Cardiology physician staff, and our referring physicians. Carlyle Fraser Heart Center (Atlanta, GA) and Positron Corp. Inc. (Houston, TX) provided financial support.
Footnotes
Received Feb. 17, 2005; revision accepted Jul. 7, 2005.
For correspondence or reprints contact: Randolph E. Patterson, MD, Emory Crawford Long Hospital, 6th Floor Medical Office Tower, Atlanta, GA 30308.
E-mail: randy_patterson{at}emoryhealthcare.org