Abstract
Soft-tissue attenuation artifacts generally appear as fixed perfusion-scan defects. Gated 99mTc-tetrofosmin SPECT may help differentiate myocardial infarction (MI) from artifacts, as fixed defects with decreased function (wall motion and thickening) probably represent MI, whereas attenuation artifacts represent preserved function. Methods: Ungated stress and gated rest 99mTc-tetrofosmin SPECT was performed on 153 consecutive patients referred for evaluation of coronary artery disease. From stress and summed gated rest images, 107 patients (70%) were identified with isolated fixed defects. The function of the defects was assessed semiquantitatively from gated stress images. The findings were correlated with clinical (history or electrocardiographic Q waves) evidence of MI. Results: Of 62 patients with fixed defects and clinical MI, 60 (97%) had an abnormal defect function. Of 45 patients with no clinical MI, 16 (36%) had decreased function of the defect, possibly indicating silent MI. In 29 of the 45 patients (64%) with no clinical MI, defect function was normal. Because most (90%) fixed defects with normal systolic function occurred in men with inferior fixed defects (87%) or women with anterior fixed defects (3%), these were most likely attenuation artifacts. By reclassifying the condition of patients with fixed defects and normal function as normal, patients with unexplained fixed defects (no clinical MI) decreased from 29% to 10%. Conclusion: Gating adds considerable value to 99mTc-tetrofosmin SPECT myocardial perfusion imaging in characterizing fixed defects and potentially improves test specificity.
Myocardial perfusion imaging is a widely used technique for the assessment of coronary artery disease (CAD). Soft-tissue attenuation in the chest produces regional inhomogeneities in the normal myocardial perfusion distribution and has been recognized as one of the most frequent causes of artifacts in myocardial perfusion imaging. If patient positioning for rest and stress acquisition is kept constant, soft-tissue attenuation artifacts appear as fixed defects. The resulting uncertainty in differentiating a fixed defect due to attenuation artifacts from myocardial infarction (MI) is an important source of false-positive scan findings, which decrease test specificity.
In the last decade, 99mTc-labeled myocardial perfusion agents have emerged as valid alternatives to 201Tl for the assessment of CAD (1). The high count-density of 99mTc-labeled myocardial perfusion agents has enabled acquisition of gated SPECT studies. Gated SPECT represents a great step forward in the evolution of functional myocardial imaging (2). Gating allows simultaneous assessment of resting ventricular function and either stress or rest perfusion distribution. Because, with 99mTc-labeled sestamibi or tetrofosmin, the tracer distribution in the myocardium is stable, spatial and temporal changes in myocardial tracer activity during the cardiac cycle reflect regional myocardial wall motion (2). More precisely, an increase in regional myocardial activity from diastole to systole is proportional to wall thickening (3). Therefore, in addition to perfusion data, gated SPECT has the potential to measure left ventricular volumes, ejection fraction, wall motion, and wall thickening (4). A fully automatic quantitative technique to measure left ventricular functional data has been developed by Germano et al. (5) and validated against a variety of techniques, such as equilibrium blood-pool studies (6), contrast ventriculography (7–9), first-pass ventriculography (8–10), MRI (11–13), and 2-dimensional echocardiography (3,14). Several studies have shown that gating may provide a valuable adjunct to 99mTc-sestamibi SPECT in characterizing fixed defects (15,16), whereas other studies have not (17). Two small studies have shown the potential of 99mTc-tetrofosmin gated SPECT to detect preserved wall motion in post-MI patients, indicating residual myocardial viability (18). Despite the large volume of data establishing in several thousand patients the prognostic utility of 99mTc-sestamibi, in the light of the current results this utility should not be empirically extrapolated to 99mTc-tetrofosmin. In fact, both compounds may differ in extraction fraction (19), heterogeneity of myocardial accumulation (20), and heart-to-liver ratios (21), which may certainly have the potential to affect not only image quality but also interpretation (19). These aspects are particularly important when performing gated SPECT, in which spatial and temporal changes in myocardial tracer activity during the cardiac cycle reflect regional myocardial wall motion.
The aims of the present study were to validate 99mTc-tetrofosmin gated SPECT wall motion analysis against echocardiography and to use these results to differentiate scars (fixed defects with decreased wall motion and wall thickening) from attenuation artifacts (which should move and thicken normally).
MATERIALS AND METHODS
Patients
We studied 153 consecutive patients who underwent gated SPECT 99mTc-tetrofosmin myocardial perfusion imaging for evaluation of suspected or known CAD. A cardiologist obtained a thorough history for every patient. A 12-lead electrocardiographic (ECG) examination was performed before the scan to evaluate the probability of a prior MI, with the conventional criterion of abnormal Q waves in 2 or more leads considered to indicate infarction.
Because coronary angiography was not performed on all patients, the true incidence of CAD is unknown. Therefore, all patients were characterized on the basis of clinical and ECG findings. Patients in whom echocardiography had shown abnormal wall motion and thickening, including cardiomyopathy, valvular disease, left bundle branch block, and paced rhythm, were excluded from the study. ECG or historical evidence of prior MI was present in 62 patients who were referred for imaging because of recurrent chest pain. Eight patients with prior transluminal coronary angioplasty and 4 patients who had undergone bypass surgery, as well as 1 patient who had undergone both bypass surgery and transluminal coronary angioplasty, were asymptomatic and referred for routine follow-up evaluation. The remaining 70 patients without a prior cardiac history were referred for the evaluation of chest pain. In that group, 42 patients (60%) had scintigraphic evidence of ischemia.
Echocardiography
In 53 of the 153 patients, 2-dimensional echocardiography was performed within 2 mo (average, 3 wk) of the gated SPECT study in the standard parasternal long-axis and short-axis views as well as in the apical 4-chamber and 2-chamber views. An experienced echocardiographer who had no knowledge of the SPECT data scored the echocardiographic findings using the 4-point scale and visually assigned segments to normal versus abnormal (hypokinesia, akinesia, dyskinesia) motility, primarily using the standard 16 segments (22). For better matching of vascular areas and more precise alignment of the gated SPECT and echocardiographic images, the segmental division of the left ventricle for gated SPECT and echocardiography was regrouped to 5 myocardial regions, that is, anterior, lateral, inferior, septal, and apical. For comparison, the gated SPECT regions were matched to the same echocardiography regions.
Stress Protocol
All 153 patients underwent a 1-d stress/rest protocol. In this protocol, 240–260 MBq of 99mTc-tetrofosmin were injected after 3 min of a 7-min infusion of intravenous adenosine at a standard rate of 140 μg/kg/min (23). No patients consumed caffeine-containing beverages or took β-adrenergic antagonists or other antiischemic medication for at least 12 h before the test.
After 45–60 min, the nongated acquisition of the stress study was performed. Four hours later, 750–800 MBq of 99mTc-tetrofosmin were injected, and the gated acquisition began 45–60 min later.
Gated SPECT
One hour after injection, gated 99mTc-tetrofosmin SPECT studies were acquired with a triple-detector camera (PRISM 3000 XP; Picker); a low-energy, high-resolution collimator; a 20% symmetric window at 140 keV; a 64 × 64 matrix; an elliptic orbit with step-and-shoot acquisition at 3° intervals over 180°; and a 20-s dwell time per stop. Acquisitions were gated at 12 frames per R-R cycle with a 20% window. For all patients, the summed nongated SPECT short-axis, vertical long-axis, and horizontal long-axis slices encompassing the entire left ventricle were first interpreted. In addition, polar maps of perfusion, wall motion, and wall thickening were produced using a commercially available software package (Cedars QGS; Cedars-Sinai Medical Center), yielding a dynamic 3-dimensional image of the left ventricle as well as numeric values of left ventricular volumes and ejection fraction (24). The function of myocardial segments with fixed defects was compared with adjacent and contralateral segments (Fig. 1). Wall motion was measured as endocardial excursion during the cardiac cycle. Regional wall thickening was assessed by image intensification from end-diastole to end-systole.
Polar map view of a normalized rest perfusion scan and the corresponding thickening assessed by gated SPECT. (A) Example of a patient with fixed inferoseptal perfusion defect but normal thickening in all segments. (B) Example of a patient with fixed inferolateral perfusion defect with congruent decreased wall thickening.
Analysis of Wall Motility
Values for wall motion and thickening of segments with a perfusion defect were expressed as a percentage of the maximal value in the polar map. With these numbers from the first 53 patients, the cutoff criterion for the best discrimination of pathologic versus normal wall motility was validated against echocardiography findings, that is, normal or pathologic wall motility. The ROC analysis versus echocardiography resulted in a cutoff value of ≤40% for wall thickening (Fig. 2A) and for wall motion (Fig. 2B). Because the area under the curve (AUC), sensitivity, and specificity were substantially greater for wall thickening (0.895, 79%, and 99%, respectively) than for wall motion (0.710, 50%, 90%, respectively), we used myocardial thickening for the analysis of the entire study.
(A) Receiver operator characteristic curve for segmental systolic wall thickening as obtained by gated SPECT versus the gold standard echocardiography. Area under the curve = 0.895. For a cutoff value of 33%, sensitivity and specificity are 79% and 99%, respectively. (B) Receiver operator characteristic curve for segmental systolic wall motion (mm) as obtained by gated SPECT versus the gold standard echocardiography. Area under the curve = 0.710. For a cutoff value of 40%, sensitivity and specificity are 50% and 90%, respectively.
Statistical Analysis
To validate the gated SPECT parameters against the echocardiography gold standard, we performed a receiver operator characteristic (ROC) analysis to obtain the cutoff value for normal versus pathologic wall. The area under the curve (AUC) was used to reflect the accuracy of the test. At the cutoff value, sensitivity and specificity were calculated.
RESULTS
Of the 153 patients, 107 (70%) demonstrated fixed defects in the nongated stress/rest images. Of these, 76 (71%) demonstrated abnormal wall thickening. Sixty of these 76 patients (79%) had historical or ECG evidence of prior MI. The other 16 (21%) with a fixed defect and abnormal function were distributed among patients who had no historical or ECG evidence of prior infarction.
In 31 of the 107 patients with fixed defects (29%), the function of the fixed defect was normal. Two of these patients (6%) had prior infarction, whereas most (94%) had no evidence of prior infarction (Fig. 3).
Schematic overview of patients with fixed defect or normal perfusion grouped according to the wall thickening finding in gated SPECT. FN = false-negative; FP = false-positive; TN = true-negative; TP = true-positive.
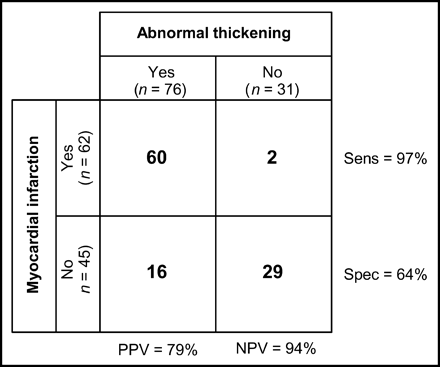
These data showed that a fixed defect with abnormal function had a positive predictive value of 79% for prior MI (Fig. 4). The negative predictive value of a fixed defect with normal function was 94% for the absence of prior infarction. The sensitivity and specificity of abnormal wall thickening for the detection of infarcted segments were 97% and 64%, respectively.
χ2 test comparing pathologic thickening vs. electrocardiographic and historical evidence of MI in patients with fixed perfusion defects. NPV = negative predictive value; PPV = positive predictive value; Sens = sensitivity; Spec = specificity. Overall agreement was 83%. The P value for the χ2 test was <0.005.
In analyzing these data differently, we observed that of 107 patients with fixed defects, only 62 had evidence of prior infarction. Thus, the specificity of a fixed perfusion defect for prior infarction was only 58%. Of these 62 patients with documented prior infarction, 60 (97%) demonstrated abnormal function on gated sestamibi SPECT. Of the 45 patients with no evidence of prior MI, 16 (36%) nonetheless demonstrated abnormal regional function. In 29 patients (64%), regional function was normal.
We investigated the possible reasons, with regard to sex and defect location, that normal function was found in areas of fixed defects for the 29 patients who had this finding and no prior MI. Of these, 1 (3%) was present in a woman and was located anteriorly. We suspected, therefore, that this defect was most likely secondary to breast attenuation artifacts. Twenty-five fixed defects with normal function (87%) occurred in the inferior wall of men. We suspected that these were most likely due to attenuation by the left hemidiaphragm. Two inferior defects with normal thickening occurred in women. Also, 1 anterior defect in a man demonstrated normal function. Overall, 90% of fixed defects with normal function could be accounted for by breast attenuation in women or diaphragmatic attenuation in men.
DISCUSSION
Myocardial perfusion imaging is a widely accepted technique for the assessment of CAD as well as for the prediction of patient outcomes (4,25–28), but its accuracy may substantially be hampered by soft-tissue attenuation artifacts. Because left ventricular functional information is available with gated SPECT, the use of wall motion and thickening for differentiating soft-tissue attenuation from true perfusion defects has been suggested, as nonviable myocardium is not expected to move or thicken normally (15).
Our study showed that wall thickening information obtained from gated SPECT is substantially more accurate (as validated against echocardiography) than is gated SPECT wall motion analysis for investigating wall motion abnormality (Fig. 2). The latter may be hampered by small-infarct tethering and by postoperative dyskinesia. Therefore, in the present study we used gated SPECT wall thickening for discriminating scars from attenuation artifacts in fixed defects. This decision was in agreement with a previous study showing that information on regional wall motion and thickening can reliably be obtained by gated SPECT (3).
In 29% of patients with fixed defects, we found normal wall thickening, indicative of viable myocardium, thus classifying the defect as false positive. Of these patients, most had no historical or ECG signs of MI (Fig. 3). Thus, the true-negative predictive value of wall thickening was 94%. In turn, 71% of patients with fixed defects had abnormal wall thickening, resulting in a negative predictive value of 79% (Fig. 4). By reclassifying the condition of patients with fixed defects and normal function as normal, patients with unexplained fixed defects (no clinical MI) decreased from 29% to 10%.
More important, the clinical relevance of the present study may be seen by the impact of gating on the specificity of 99mTc-tetrofosmin in diagnosing CAD. Without gating, all fixed defects are interpreted as infarction, indicating CAD, resulting in a false-positive rate of 20% in our entire study population. The use of thickening information to help differentiate scars from attenuation artifacts reduced the adjusted false-positive rate to 10%. To our knowledge, our study was the first to confirm, in a large study population, that results with 99mTc-tetrofosmin are similar to those previously reported for 99mTc-sestamibi (15,29,30) and 201Tl (14). Interestingly, only 4% of all patients with normal perfusion had false-positive abnormal thickening, underlining the reliability of the technique.
In our study, 90% of the false-positive fixed defects showed a pattern with high likelihood of being soft-tissue artifacts (inferior abdominal attenuation for men, and anterior breast attenuation for women). Interpreting physicians who are aware of these problems may try to overcome them by interpreting some defects as artifacts. This attempt, however, may be made at the cost of confidence. In fact, adding gating information has decreased the number of borderline interpretations in a previous study, increasing the numbers in the definitely-normal and definitely-abnormal categories (16). By contrast, gated SPECT had little influence in another study (17), as the readers were committed to—although not entirely confident in—a definitive diagnosis before evaluating the gating.
The study had some limitations. A cardiologist obtained a thorough history for each patient, and a 12-lead ECG examination was performed to evaluate the possibility of prior MI. This, however, might not have provided or excluded definitive evidence of the presence or absence of MI in the study population, as historical and electrocardiographic criteria for MI have limited accuracy. In an autopsy series, the sensitivity of electrocardiographic Q waves for MI was as low as 61% (31). The specificity may be inversely affected by small inferior Q waves in patients without infarction and by poor R-wave progression not always indicative of anterior MI. Although we used pharmacologic stress, and although imaging was performed 1 h after injection, a chance remains that stunning may have contributed to false-positive findings in some patients. However, as the vasodilator adenosine rarely induces an ischemic reaction massive enough to cause stunning lasting as long as 1 h, false-positive findings due to stunning appear unlikely.
In 3% of patients, we observed normal wall thickening despite prior MI. This thickening may have been due to the smallness of the infarct or to a nontransmural infarct distribution. An alternative is that Q waves simply are not 100% specific for transmural infarction.
As can be seen in Figure 3, 45 (true negatives and false positives) of the 107 patients with a fixed defect had no evidence of prior MI. Nevertheless, in 16 (false positives) of these 45 patients (36%), we found regional thickening abnormalities. These defects could have represented segments affected by resting ischemia or a history of clinically silent non-Q-wave MI. Because both regional perfusion defects and wall motion abnormalities have been described in patients with cardiomyopathy and valvular disease, such patients were not included in the present study. In thin (infarcted) ventricular segments, partial-volume effects may contribute to overestimation of impaired wall thickening. This effect is probably less pronounced in attenuation artifacts with normal wall thickness and, thus, might have only minimally contributed to the false-positive findings of our study.
CONCLUSION
Obviously, gating provides functional information not available in static perfusion images. Our study shows that wall thickening is the best parameter derived from gating 99mTc-tetrofosmin SPECT, which increases test specificity for CAD assessment by allowing better differentiation of scars from attenuation artifacts when interpreting the cause of fixed defects.
Acknowledgments
We are grateful to our head radiographer, Merill Griff, for his excellent technical assistance. One of the authors was funded by a grant from the Swiss National Science Foundation (SNSF Professorship) and supported by a grant from the EMDO STIFTUNG Zurich and the Radiumfonds Zurich.
Footnotes
Received Sep. 25, 2003; revision accepted Dec. 29, 2003.
For correspondence or reprints contact: Philipp A. Kaufmann, MD, Cardiovascular Center C NUK 32, Nuclear Cardiology Section, University Hospital, Ramistrasse 100, CH-8091 Zurich, Switzerland.
E-mail: pak{at}usz.ch