Abstract
Although a number of different strategies for labeling peptides with 99mTc have been developed, only a few studies have compared the in vivo properties of 99mTc when attached to different chelators. Furthermore, these comparisons are usually in mice, whereas results obtained in nonhuman primates may be expected to be more relevant to the clinical situation. Methods: We evaluated the influence of 4 common chelators on the biodistribution in monkeys of 99mTc-labeled HNE-2, a 6.7-kDa peptide being investigated as an inflammation/infection imaging agent. The peptide was conjugated with the N-hydroxysuccinimide ester of mercaptoacetyltriglycine (MAG3), mercaptoacetyltriserine (MAS3), hydrazinonicotinamide (HYNIC), and the cyclic anhydride of diethylenetriaminepentaacetic acid (DTPA). After radiolabeling, each peptide was administered intravenously to rhesus monkeys with a Staphylococcus aureus–induced focal inflammation/infection. Results: Quantification of radioactivity accumulation by regions of interest over 3 h after administration in monkeys showed important differences among labeling methods: For example, at 3 h, kidney accumulation varied in percentage injected dose per organ (%ID per organ) from 31 %ID per organ (HYNIC) to 18 %ID per organ (MAG3), whereas liver varied from 7.8 %ID per organ (MAG3) to 2.8 %ID per organ (MAS3). Radioactivity accumulation in the lesion was independent of labeling method. These organ accumulations were compared with that obtained earlier in mice by sacrifice and dissection also at 3 h and at the same administered dosage. In the rodent, kidney levels varied from 45 %ID per organ (HYNIC) to 12 %ID per organ (MAS3) and liver levels varied from 6.5 %ID per organ (DTPA) to 2.0 %ID per organ (MAS3). Conclusion: In agreement with previous work from this laboratory and elsewhere, the method of radiolabeling had an important effect on the biodistribution of 99mTc. Furthermore, although biodistribution results in mice should be used with caution to predict biodistributions in primates, in major organs, these results in mice and monkeys were similar.
The use of small, labeled peptides as radiopharmaceuticals is an area that has seen a growing number of applications in recent years. Peptides have been labeled with a number of isotopes, although 99mTc remains one of the most useful for single-photon diagnostic imaging. 99mTc-Labeling strategies for peptides have been reviewed extensively (1–3), and this laboratory has investigated various 99mTc-labeling strategies from a growing list of bifunctional chelators (4–6). As this laboratory and others have shown, the method selected for 99mTc labeling can have a pronounced effect on the properties of the label in vitro and in vivo (7–11). Previously, we reported on 2 similar human neutrophil elastase inhibitor peptides, EPI-HNE-2 (HNE-2) and EPI-HNE-4 (HNE-4) (7,12), labeled with 99mTc using 3 bifunctional chelators: the N-hydroxysuccinimide ester of S-acetyl mercaptoacetyltriglycine (MAG3) (4), the cyclic anhydride of diethylenetriaminepentaacetic acid (DTPA) (13), and the N-hydroxysuccinimide ester of hydrazinonicotinamide (HYNIC) (14). On the basis of those results, we concluded that it may be important to compare chelators and labeling methods before selecting a 99mTc-labeling method for any particular peptide.
In this investigation, we describe a biodistribution study using an existing nonhuman primate model for in vivo inflammation/infection imaging (15). The peptide HNE-2 was radiolabeled with 99mTc using 1 of 4 bifunctional chelators: N-hydroxysuccinimidyl (NHS)-S-acetyl-MAG3, NHS-S-acetylmercaptoacetyltriserine (MAS3) (5), NHS-HYNIC (with tricine as coligand), and the cyclic anhydride of DTPA. Previously we used these same 4 methods to prepare 99mTc-radiolabeled HNE-2 for studies in healthy mice. The results obtained in both species are now compared.
MATERIALS AND METHODS
Standard chemicals were obtained from various suppliers and used without purification. 99mTc-Pertechnetate was obtained from a 99Mo-99mTc radionuclide generator (Dupont Pharma Radiopharmaceuticals, Billerica, MA). The peptide HNE-2 was a gift from Dyax Corp. (Cambridge, MA) and was selected from a phage library as a potent (inhibition constant, 2 pmol/L) neutrophil elastase-specific inhibitor (12). The native HNE-2 has a molecular weight of 6.7 kDa and 2 lysines plus the terminal amine available for conjugation. The synthesis of NHS-MAG3 (4) and NHS-HYNIC (14) were as described. The NHS-MAS3 synthesis and its use as an alternative to NHS-MAG3 also has been described (5).
Conjugation and Radiolabeling
NHS-MAG3 and NHS-MAS3.
The conjugation and radiolabeling with 99mTc of MAG3-conjugated peptides has been described (16). Briefly, for conjugation with NHS-MAG3 the HNE-2 peptide was first prepared at a concentration of 5 mg/mL in 0.1 mol/L N-(2-hydroxyethyl)piperazine-N′-(2-ethanesulfonic acid (HEPES) buffer, pH 8.0, to which a fresh 10 mg/mL solution of NHS-MAG3 in dry dimethylformamide (DMF) was added dropwise with agitation. The final MAG3-to-peptide molar ratio was 3:1, and the volume of DMF added was always <10% of the total volume. The reaction mixture was then incubated at room temperature for 30–60 min before purification on a 0.7 × 20 cm P4 size-exclusion open column (BioRad, Hercules, CA) with 0.25 mol/L ammonium acetate, pH 5.2, eluant. Fractions were collected and quantitated by ultraviolet (UV) absorbance at 280 nm (U-2000; Hitachi Instruments, Inc., Danbury, CT) using an extinction coefficient of 0.89 for HNE-2.
For radiolabeling, the conjugated peptide was diluted to approximately 0.4–0.5 mg/mL with 0.25 mol/L ammonium acetate, pH 5.2. To 0.25 mL of the coupled peptide solution was added an aliquot of sodium tartrate prepared to 50 mg/mL in 0.5 mol/L sodium bicarbonate, 0.25 mol/L ammonium acetate, and 0.175 mol/L ammonium hydroxide buffer, pH 9.2, for a final tartrate concentration of 7 μg/mL (for labeling at pH 7.6). After adding approximately 111 MBq 99mTc-pertechnetate generator eluant, 7 μL of a fresh solution of SnCl2·2H2O (1 mg/mL in 10 mmol/L HCl) were added. The solution was then incubated at room temperature for 30–60 min before purification over the P4 column with 50 mmol/L phosphate-buffered saline (PBS), pH 7.2, as eluant. Those fractions with the highest radioactivity were pooled and the protein concentration was determined by UV absorbance at 280 nm. The sample was analyzed for radiochemical purity using a C18 Sep-Pak mini cartridge (Waters, Milford, MA) as follows. After preconditioning with 10 mL ethanol followed by 10 mL water, an aliquot of labeled peptide sample was applied and the column was washed with 5 mL 1 mmol/L HCl to elute 99mTc-tartrate and 99mTc-pertechnetate. The labeled peptide was then recovered with a 1:1 solution of ethanol and saline, and the radiochemical purity was calculated. Finally, for administration, the labeled peptide was sterilized by passage through a 0.22-μm filter (Gelman Sciences, Ann Arbor, MI). The conjugation and radiolabeling of the peptide with NHS-MAS3 was identical to that described above for MAG3.
Cyclic Anhydride of DTPA.
The DTPA-conjugated peptide was prepared as follows. To a 10 mg/mL solution of the peptide in water was added an equal volume of 0.2 mol/L HEPES buffer, pH 8.0. A suspension of the DTPA cyclic anhydride (Sigma Chemical Co., St. Louis, MO) in DMF (10 mg/mL) was then added dropwise with agitation to a final DTPA-to-peptide molar ratio of 3:1. After 30 min at room temperature, the coupled peptide was purified on the P4 column as described above. For radiolabeling with 99mTc, 0.05 mL DTPA-peptide solution (∼2.0 mg/mL in 0.25 mol/L ammonium acetate buffer, pH 5.2) was added to 18 μL of a buffer consisting of 0.5 mol/L sodium bicarbonate, 0.25 mol/L ammonium acetate, and 0.175 mol/L ammonium hydroxide, pH 9.2. To this was added approximately 148 MBq 99mTc-pertechnetate (20–40 μL) followed immediately by addition of 6–12 μL of a fresh solution of SnCl2·2H2O (1 mg/mL in 10 mmol/L HCl). After 30 min at room temperature, the preparation was purified over the P4 column with 50 mmol/L PBS (pH 7.2) eluant. Analysis and sterilization for administration were similar to those described above for MAG3.
NHS-HYNIC.
The peptide was conjugated with NHS-HYNIC using a 3:1 HYNIC-to-peptide molar ratio as described (7) followed by purification on the P4 column as described above. Approximately 20 μL of a 0.1 mg/mL tricine solution in water was added to approximately 0.1 mg of the HYNIC-peptide in 0.1 mL of 0.25 mol/L ammonium acetate, pH 5.2, to which was added approximately 111 MBq 99mTc-pertechnetate generator eluant, followed by addition of 6 μL fresh SnCl2·2H2O (1 mg/mL in 10 mmol/L HCl) solution. After incubation at room temperature for 30–60 min, the labeled peptide was purified over the P4 column with 50 mmol/L PBS (pH 7.2). Analysis and preparation for administration were similar to those described above for MAG3.
Serum Stability
Size-exclusion high-performance liquid chromatography (HPLC) analysis was used to estimate the stability of 99mTc on each peptide preparation toward incubation at 37°C in fresh human serum. All radiolabeled peptides were analyzed by size-exclusion HPLC using a 1 × 30 cm Superdex-peptide column (Pharmacia, Piscataway, NJ), with 0.1 mol/L sodium phosphate, pH 7.0, as eluant and with in-line radioactivity and UV detection. The labeled peptides were added to 37°C serum at a concentration of approximately 1–5 μg/mL, and samples were removed for analysis at various times from 5 min to 24 h. Recovery of radioactivity was routinely determined. A shift of the radioactivity profile to higher molecular weight could signify serum protein binding, whereas the presence of lower-molecular-weight peaks could signify a breakdown to labeled catabolites or dissociation of the label.
Mouse Biodistribution
All animal studies were performed with the approval of the Institutional Animal Care and Use Committee. In groups of 5, normal CD-1 male mice (∼28 g; Charles River, Wilmington, MA) were injected through a tail vein with 0.1 mL 50 mmol/L PBS containing 1–2 μg (370–740 kBq) labeled peptide. At 3 h, animals were anesthetized with metofane (Schering-Plough, Omaha, NE) and killed by cervical dislocation. Whole blood was collected and tissues of interest were removed for counting in a NaI(Tl) well counter along with a standard of the injectate.
Inflammation Model
Five rhesus macaque monkeys (Michigan Biologic Products Institute, Lansing, MI), weighing 10–15 kg each, were used. The inflammation/infection model has been described (15). In brief, the inflammation was induced by the subcutaneous administration of a uniform suspension of Staphylococcus aureus cell products (Sigma; 6 mg/0.4 mL sterile saline), to which was added 50 mg arachidonic acid (Sigma). The animals were injected intramuscularly with 20 mg/kg of ketamine (Fort Dodge Laboratories, Inc., Fort Dodge, IA). The target area (lower back) was shaved and swabbed with alcohol, and the mixture of S. aureus and arachidonic acid was administered subcutaneously. The lesion typically resolved within a few days and the animals showed no signs of systemic infection. The radiolabeled peptide was administered 1 d after infection.
Imaging Protocol
Scintigrams were acquired on a portable large field-of-view scintillation camera (Elscint, Hackensack, NJ) equipped with a parallel-hole, low-energy collimator and an Elscint APEX F1 computer. Animals were positioned posteriorly on the collimator, and radioactivity was counted for 30–480 s using a 512 × 512 matrix with a 20% energy window set at 140 keV. Before imaging, each animal was anesthetized with ketamine and intubated with an uncuffed tracheal tube (Mallinckrodt Medical Inc., St. Louis, MO). Animals received 2% isoflurane (Abbott Laboratories, North Chicago, IL) in oxygen to maintain anesthesia.
A total of 10 imaging studies were performed, 3 each with MAG3 and MAS3 and 2 each with DTPA and HYNIC. Assignment of monkeys and labeling method were random. The dosage of peptide ranged between 42 and 67 μg labeled with 26–39 MBq. The injectate was administered as a bolus through an in-line catheter in a right arm vein and was followed by a saline flush. Images were acquired immediately and for the next 4 h. Approximately 10 min after injection of the labeled peptide, and throughout the imaging period, lactated Ringer’s solution (Baxter Health Care, Deerfield, IL) was infused through the catheter at a rate of approximately 24 mL/h to maintain electrolyte balance.
For analysis of the whole-body images, regions of interest were placed about the left and right kidneys, the liver, the lesion (lower back), and a contralateral area of the lower back for background subtraction. Counts were recorded without absorption correction. An estimate of the radioactivity in becquerels within each region was obtained by applying the counts to a calibration curve of counts per minute versus becquerels obtained using standard solutions of 99mTc. Results were then normalized to the radioactivity administered.
Plasma Collection
Blood samples of approximately 0.2 mL were obtained by a direct needle stick, transferred to a Vacutainer tube (Becton-Dickinson, Franklin Lakes, NJ) coated with ethylenediaminetetraacid acid, and immediately placed on ice for plasma analysis. After separation, samples of plasma were counted for radioactivity against a standard of the injectate so that the results could be presented as percentage injected dose (%ID) per milliliter. Samples were also analyzed by size-exclusion HPLC as described above. Plasma not analyzed immediately was stored at 4°C.
RESULTS
Radiolabeling
Radiolabeling efficiencies ranged between 44% and 85% and were independent of the method and the chelator used. After P4 column purification, the average radiochemical purity for all preparations by Sep-Pak analysis was 88.5% ± 4.6% (mean ± SD). No attempt was made to maximize specific activity.
In Vitro Serum Incubation
Size-exclusion HPLC radiochromatograms of in vitro 37°C serum incubations for HNE-2 radiolabeled through MAG3 and DTPA at pH 7.6 and HYNIC have been presented previously (7). The radiochromatograms obtained in this investigation for HNE-2 radiolabeled through MAS3 were similar. After only 5 min of incubation in serum, radioactivity associated with higher-molecular-weight peaks was evident for each preparation, probably as a result of protein binding. In each case, the radioactivity associated with higher-molecular-weight peaks increased with additional incubation time. Distinct minor species of low molecular weight became apparent in the 1-h chromatograms in all cases. Species eluting in these later fractions have been identified previously as radiolabeled cysteine resulting from the transchelation of the label from peptide to endogenous cysteine (17,18), as may be the case here.
Biodistribution
Plasma clearance curves in monkeys for 99mTc radiolabeled to HNE-2 are presented in Figure 1. These data are averages obtained from 2 or 3 studies with each chelator. Plasma clearance rates were rapid and were similar for 3 chelators: MAG3, MAS3, and HYNIC. Circulating levels of radioactivity decreased to <0.1 %ID by 30–40 min after administration, and circulating levels at 3–4 h were 0.02–0.04 %ID. However, when HNE-2 was labeled using DTPA, plasma clearance was approximately 2-fold slower.
Clearance from plasma of 99mTc labeled to HNE-2 using MAG3 (•), MAS3 (⧫), DTPA (▪), and HYNIC (▴).
Figure 2 shows HPLC radiochromatograms of 99mTc in plasma collected from monkeys at 5–60 min. For comparison, a radiochromatogram of each labeled HNE-2 in saline is also presented (Fig. 2A). As early as 5 min (Fig. 2B), multiple high-molecular-weight peaks are clearly evident in the MAG3, MAS3, and HYNIC samples. By 30 min, for all 4 chelators (Fig. 2C), about half of the radioactivity in plasma eluted with an earlier retention time than did the labeled peptide in saline, most probably because of serum protein binding.
Size-exclusion HPLC radiochromatograms of 99mTc labeled to HNE-2 using 4 chelators in saline (A) and in plasma collected from monkeys at 5 min (B), 13–30 min (C), and 60 min (D).
Average radioactivity levels in liver from 0 to 4 h are presented in Figure 3. Important differences in liver activity are evident among the various labeling methods, with the highest levels obtained using MAG3, whereas MAS3 provided the lowest levels by a factor of approximately 3. Average radioactivity levels in kidneys are shown in Figure 4. Kidney levels for HYNIC and MAS3 were indistinguishable and were nearly 2-fold higher than those for DTPA and MAG3.
Radioactivity clearance in liver (%ID) over 4 h for HNE-2 labeled using MAG3 (•), MAS3 (⧫), DTPA (▪), and HYNIC (▴). Data points represent averages (n = 2 or 3) for each chelator.
Radioactivity clearance in kidneys (%ID) over 4 h for HNE-2 labeled using MAG3 (•), MAS3 (⧫), DTPA (▪), and HYNIC (▴). Data points represent averages (n = 2 or 3) for each chelator.
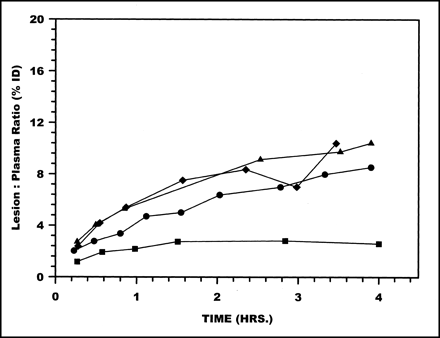
Radioactivity accumulations in the lesion showed no differences among the labeling methods. However, as shown in Figure 5, when presented as lesion-to-plasma ratios (i.e., %ID in the lesion compared with plasma radioactivity per milliliter), DTPA showed the lowest ratio at all time points.
Radioactivity ratios between lesion (%ID) to plasma (%ID/mL) over 4 h for HNE-2 labeled using MAG3 (•), MAS3 (⧫), DTPA (▪), and HYNIC (▴).
Comparative Biodistributions
Table 1 compares biodistribution in monkeys and mice of 99mTc in %ID per total organ at 3 h after IV administration of radiolabeled HNE-2. The dosage was 3–67 μg/kg in mice, and for monkeys the dosage was 3–7 μg/kg. Results in Table 1 for radiolabeled MAG3, HYNIC, and DTPA in mice have been reported previously (7). Data for the monkey are based on in vivo imaging data for which counts were obtained from within regions of interest placed about the liver, heart, kidneys, and spleen. For the mouse, entire organs were removed for counting in a well counter.
Biodistribution of Labeled Peptides in Monkeys and in Mice
Organ radioactivity levels shown in Table 1 are generally higher in monkeys compared with mice. Contributions from overlying and underlying tissues can be expected to result in an overestimate of in vivo organ radioactivity levels. An overestimate would be expected to be most serious for those organs with lower levels of accumulations, such as heart and spleen. These effects are evident in the data shown in Table 1.
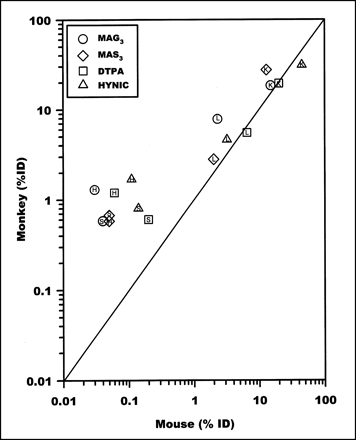
Figure 6 illustrates the correlation between the mouse and monkey data of Table 1. A logarithm–logarithm scale is used to accommodate the wide dynamic range of these data. The solid line in Figure 6 depicts the behavior expected if the relationship between the 2 datasets was exact. Whenever the mouse values are relatively high (>1%), a good correlation is apparent despite the differences in dosage. Whenever the mouse values are less than approximately 1% (e.g., heart and spleen), the correlation between the mouse and monkey datasets is less exact, presumably because of contributions from background radioactivity levels to the primate measurements. Clearly, the exact size and position of the area of interest will influence the magnitude of the background contribution.
Logarithm–logarithm correlation plot of %ID per organ in monkey and mouse in kidney (K), liver (L), heart (H), and spleen (S). Data are taken from Table 1.
A composite of scintigraphic images is shown in Figure 7. The images are from 3 h after administration, with 1 example shown for each of the 4 99mTc-chelate preparations. The images are shown to emphasize the difference in clearance and organ accumulation of the 4 chelate complexes. For example, liver is highest with MAG3 and lowest with MAS3.
Composite of scintigraphic images in monkeys 3 h after administration, with 1 example shown for each of 4 99mTc-chelate complexes.
DISCUSSION
It has become evident that for peptides, possibly because of their relatively small size, the choice of chelating agent can influence the in vitro properties and the biodistribution of 99mTc (7–11). Previously, this laboratory showed in the same monkey model that 99mTc-MAG3-HNE-2 localized rapidly and specifically in sites of infection/inflammation (15). The principal aim of this study was to use the nonhuman primate infection model to compare the properties of 99mTc attached to HNE-2 by 4 different chelating agents. A second aim was to compare the biodistribution of 99mTc in major organs between monkeys and healthy mice administered radiolabeled peptides differing only in the nature of the chelating agent.
In a previous investigation (7), size-exclusion HPLC analysis of radiolabeled peptides incubated in serum in vitro showed evidence for serum protein binding within 5 min of incubation. Serum protein binding is also the most likely explanation for the shift in radioactivity to earlier retention times seen in the radiochromatograms of plasma samples obtained from monkeys in this investigation (Fig. 2). Distinct low-molecular-weight species were not prominent in any of the radiochromatograms, although DTPA showed a small broad peak at longer elution times. Therefore, the slower plasma clearance of DTPA relative to that of the remaining chelators (Fig. 1) cannot be attributed either to association with serum proteins or to label dissociation, but probably reflects a chelator-specific influence on clearance.
Major chelator-related differences in the biodistributions were observed in liver and kidneys. HNE-2 labeled using HYNIC and MAS3 showed the lowest accumulations in liver (Figs. 3 and 7) and the highest accumulations in kidneys (Fig. 4). Relative levels of radioactivity in these 2 organs may indicate the relative contributions of renal and hepatobiliary routes of clearance. For small peptides, the relative overall hydrophobicity of the peptide can substantially influence the route of biologic clearance (2). In general, hepatobiliary clearance is the major route for more hydrophobic peptides, such as formyl-Met-Leu-Phe (11), whereas more hydrophilic peptides, such as free MAG3 (19), are cleared though renal filtration and excretion. The peptide HNE-2 contains a very hydrophobic solvent–exposed stretch of 5 amino acids (Ile-Ala-Phe-Phe-Pro) that confers the high affinity and specificity to human neutrophil elastase (12). It seems likely that the presence of this hydrophobic patch on the surface of the HNE-2 molecule influences the distribution of the radiolabeled compound between routes of clearance in vivo. The presence of 1 or more chelating groups of differing polarities coupled to HNE-2 may further modulate this distribution. In particular, the change in chelator from MAG3 to the more polar, but otherwise similar, MAS3 is accompanied by substantial decreases in liver and concomitant increases in kidney accumulations of radioactivity.
Accumulations of radioactivity in the lesion were similar for all 4 labeled HNE-2s. These in vivo results are therefore consistent with in vitro measurements showing that the methods of conjugating the peptide with bifunctional chelators do not influence the activity of the molecule toward human neutrophil elastase (15). However, when normalized to circulating plasma radioactivity, the DTPA-HNE-2 complex, relative to the other 3, showed a substantially lower level of target accumulation (Fig. 5). The DTPA-HNE-2 complex was also distinct among the 4 chelator-peptide complexes in showing a slower rate of plasma clearance and higher levels of circulating radioactivity at all times.
Overall, our results suggest that, among those considered, MAS3 is the preferred method for labeling HNE-2. This chelator is readily synthesized using inexpensive reagents and can be coupled with good efficiencies to HNE-2. The MAS3-HNE-2 complex retains the physicochemical stability properties of the unmodified molecule (A.C. Ley, unpublished data, 1999) along with accumulation in the intended target, human neutrophil elastase. The complex can be effectively and stably radiolabeled with 99mTc without alteration of its properties. In vivo, the radiolabeled complex clears rapidly from circulation, accumulates rapidly and specifically at sites of infection/inflammation, and shows minimal levels of nonspecific organ accumulation.
The correlation between the mouse and monkey biodistribution datasets, shown in Figure 6, suggests that biodistribution data in mice are loosely predictive of the results in primates. As discussed above, the correlation between the datasets is less exact for low levels of organ radioactivity accumulation where, presumably, the methodology used to obtain the monkey data resulted in substantial overestimates of organ accumulations in some cases because of inclusion of background radioactivity. If so, extrapolation of the mouse data to primates may be more valid than that indicated by Figure 6 because the discrepancies may be the result of artifacts related to the noninvasive method used to estimate biodistributions in the monkey. Regardless, for most of the kidney and liver data listed in Table 1, the mouse and monkey accumulation levels agree to within a factor of 1.5. The 2 notable exceptions are the MAG3 liver data and the MAS3 kidney data, where the 2 sets differ by factors of 2.5 and 2.2, respectively. However, the mouse data accurately predicted the large 3- to 10-fold differences in specific organ accumulation between the liver and kidney data in monkeys. Thus, such large differences in accumulation of radioactivity in specific organs for specific radiolabeled chelator-peptide complexes in mice may be predictive of similar large differences in the pharmacokinetics and biodistribution of the complexes in humans.
CONCLUSION
In this investigation 1 peptide was radiolabeled with 99mTc using 4 different chelators and tested in a nonhuman primate infection/inflammation model. Because we and others have previously observed an influence of labeling methods on the biodistribution of 99mTc in mice when attached to small molecules such as HNE-2, an influence of labeling method on biodistribution was expected and was again observed, now in monkeys. Accordingly, we again conclude that the method selected for labeling peptides is likely to influence the properties of the radiolabel. More surprisingly perhaps, we observed a weak but possibly useful correlation between radioactivity accumulations in several organs, especially liver and kidneys, between monkeys and mice. We conclude that biodistribution studies of different labeling methods in mice may provide an indication of the influence of these labeling methods on the biodistribution in monkeys, at least in major organs.
Acknowledgments
The authors are grateful to Dyax Corporation for providing the HNE-2 and for providing partial financial support for this research.
Footnotes
Received Apr. 6, 2001; revision accepted Aug. 20, 2001.
For correspondence or reprints contact: Mary Rusckowski, PhD, Division of Nuclear Medicine, Department of Radiology, University of Massachusetts Medical School, 55 Lake Ave. N., Worcester, MA 01655-0243.