Abstract
The aim of this retrospective study was to evaluate the detection rate of Glu-NH-CO-NH-Lys-(Ahx)-[68Ga(HBED-CC)] (68Ga-PSMA ligand; PSMA is prostate-specific membrane antigen) PET/CT in patients with biochemical recurrent prostate cancer defined by Phoenix criteria after external-beam radiotherapy or brachytherapy as primary treatment. Methods: One hundred eighteen patients with a median prostate-specific antigen (PSA) of 6.4 ng/mL (range, 2.2–158.4 ng/mL; interquartile range, 4.2–10.2 ng/mL) were finally eligible for this retrospective analysis. Seventy-seven and 41 patients had been treated by external-beam radiotherapy or brachytherapy, respectively. Of the 118 patients, 45 were receiving androgen-deprivation therapy (ADT) within at least 6 mo before the PET/CT. The detection rates were stratified by PSA. The influence of primary Gleason score and ADT was assessed. Relationships between SUV and clinical as well as pathologic features in patients with positive findings were analyzed using univariate and multivariable linear regression models. Results: One hundred seven of 118 patients (90.7%) showed pathologic findings indicative for tumor recurrence in 68Ga-PSMA ligand PET/CT. The detection rates were 81.8% (36/44), 95.3% (41/43), and 96.8% (30/31) for PSA of 2 to <5, 5 to <10, and ≥10 ng/mL, respectively (P = 0.0377). 68Ga-PSMA ligand PET/CT indicated local recurrence in 68 of 107 patients (63.5%), distant lesions in 64 of 107 patients (59.8%), and local recurrence as well as distant lesions in 25 of 107 patients (23.4%). The detection rate was significantly higher in patients with ADT (97.7%) versus without ADT (86.3%, P = 0.0381), but independent from primary Gleason score ≥ 8 (92.0%) versus ≤ 7 (90.2%, P = 0.6346). SUVmax and SUVmean were significantly associated with PSA and ADT (P = 0.018 and 0.004 for SUVmax, respectively; P = 0.025 and 0.007 for SUVmean, respectively). Conclusion: 68Ga-PSMA ligand PET/CT demonstrates high detection rates in patients with biochemical recurrence of prostate cancer after primary radiation therapy. The detection rate was positively associated to increasing PSA as well as concomitant ADT. 68Ga-PSMA ligand PET/CT enables discrimination of local versus metastatic disease and thus might have a crucial impact on further clinical management. A major limitation of this study is the lack of histopathologic proof in most patients.
The most common approaches in the primary treatment of prostate cancer (PC) are radical surgery, external-beam radiation therapy (EBRT), brachytherapy (without or in combination with EBRT), or androgen-deprivation therapy (ADT) (1). After radiation therapy (RT) as the primary treatment of PC, a prostate-specific antigen value (PSA) of 2 ng/mL above the PSA nadir represents biochemical recurrence (BCR) defined by Phoenix criteria, which are the current standard of reference for the definition of BCR after primary RT (2). Biochemical failure is seen in 10%–60% of patients after EBRT, depending on pretreatment risk factors and on the radiotherapy technique used (3). After brachytherapy, BCR after 5 and 10 y was reported to range from 7% to 29% and from 15% to 35%, respectively (1,4). Monitoring of PSA is a reliable and cost-effective way to detect disease relapse. However, it cannot differentiate between local, locoregional, or systemic recurrence. Imaging modalities such as bone scintigraphy and CT exhibit considerable limitations in the setting of PSA < 10 ng/mL and may show the site of recurrence only in patients with fast PSA kinetics (PSA velocity > 2 ng/mL per year) or higher PSA values (>20 ng/mL) (5–7). By contrast, PET/CT with 11C-labeled choline derivatives in patients with BCR at low PSA values after EBRT has proven to be a valuable tool (8).
The recent introduction of Glu-NH-CO-NH-Lys-(Ahx)-[68Ga(HBED-CC)] (68Ga-PSMA ligand; PSMA is prostate-specific membrane antigen) as an extracellular PSMA inhibitor for PET imaging demonstrated excellent results, especially for patients with BCR. It showed markedly improved detection rates in direct head-to-head comparison or compared with data from literature (9,10). Most recently, Perera et al. presented a review of 68Ga-PSMA ligand PET, demonstrating a pooled detection rate of 76% for 68Ga-PSMA ligand PET/CT (11), considerably exceeding the pooled 62% detection rate for 11C-choline PET (12). However, most 68Ga-PSMA ligand PET/CT studies have either evaluated an inhomogeneous patient population, including predominantly patients showing BCR after radical prostatectomy and including also a low proportion of patients after EBRT as well as progressive disease, or completely focused on the group of patients after radical prostatectomy (10,13,14).
To the best of our knowledge, so far no study has been published focusing on the clinical performance potential of 68Ga-PSMA ligand PET in recurrent PC patients after primary curative intended RT alone. Because localization of relapse in BCR after primary RT is challenging, imaging plays a crucial role in further therapy stratification. Therefore, the purpose of our study was to evaluate the detection rate of 68Ga-PSMA ligand PET/CT and to compare it with the results of primary histologic differentiation (Gleason score [GS]) and ADT in a large population of patients with BCR according to the Phoenix criteria after primary treatment with EBRT or brachytherapy.
MATERIALS AND METHODS
Patients
One hundred eighty-three patients who underwent 68Ga-PSMA ligand PET/CT imaging for recurrent PC after primary RT were extracted from the institutions’ database (November 2012 to March 2016). Subsequently, patients in whom BCR according to the Phoenix criteria was not fulfilled were excluded from the study. BCR is defined as a PSA rise by 2 ng/mL or more above the nadir PSA after RT. Further exclusion criteria were salvage radical prostatectomy, transurethral resection of the prostate, cryosurgical ablation of the prostate, high-intensity focused ultrasound, irreversible electroporation, second-line antihormonal therapy, chemotherapy, and bone-targeted therapy with 223Ra (Fig. 1). In total, 118 patients were enrolled in this retrospective study. Details on patient characteristics are summarized in Table 1.
Flowchart of patient selection. * = transurethral resection of prostate; ** = high-intensity focal ultrasound; # = irreversible electroporation.
Patient Characteristics
All patients signed a written informed consent form for the purpose of anonymized evaluation and publication of their data. All reported investigations were performed according to the principles of the Helsinki Declaration and to national regulations. The study was approved by the Ethics Committee of the Technical University Munich (permit 5665/13).
Imaging and Interpretation
A detailed description of 68Ga-PSMA ligand and imaging parameters is available as supplemental materials (available at http://jnm.snmjournals.org). All PET/CT images were interpreted by 1 board-certified nuclear medicine physician and 1 board-certified radiologist in consensus. All lesions suggestive for recurrent PC were noted and grouped with respect to their localization into local recurrence, lymph node metastases, bone metastases, and other metastases. Imaging findings were validated in 35.5% (38/107) of patients. Further details on the validation criteria are provided as supplemental materials.
In PET, any focal uptake higher than background and not associated with physiologic uptake was judged as tissue suggestive of malignancy. For quantitative assessment, only the highest SUV was noted in each suggestive anatomic field. To calculate SUVs, an isocontour volume of interest including all voxels above 50% of the maximum was created, covering the whole lesion volume, as performed recently (15). Within all volumes of interest, mean and maximum SUVs were measured. For CT, any distinct sclerotic lesion not being associated with degenerative changes and any small lung lesion not being related to inflammatory changes or associated with typically subpleural intrapulmonary lymph nodes below the level of the carina (16) were considered as positive. Criteria for interpretation of 68Ga-PSMA ligand PET/CT have been recently published (17).
Statistical Analysis
The detection rate was plotted against the absolute PSA value. Two-sided χ2 tests to evaluate differences between single groups and Mann–Whitney U tests to evaluate differences concerning PSA values were used. Univariate and multivariable linear regression models were fit to the data to assess the association between SUV (lesion with highest SUVmax and SUVmean) and PSA, initial PSA (iPSA), body mass index, age, injected activity, acquisition time, GS (GS ≥ 8 vs. ≤ 7), ADT (with and without ADT), and the type of RT. SUVs were logarithmized to account for skewed distributions. A P value of less than 0.05 was considered significant. Statistical analyses were done with software (PRISM 6 [GraphPad]; MedCalc, version 16.8 [MedCalc]; and SPSS Statistics, version 23 [IBM]).
RESULTS
Detection Efficacy
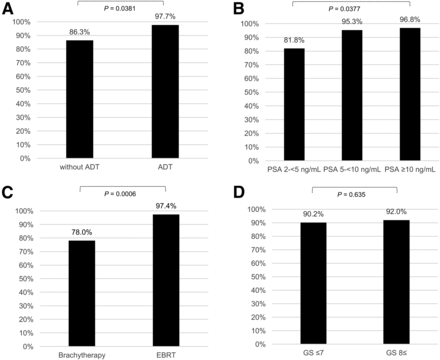
68Ga-PSMA ligand PET/CT showed pathologic findings suggestive for recurrent PC in 107 of 118 (90.7%) patients. With respect to the PSA value, the detection efficacy was 81.8% (36/44) for a PSA of 2 to <5 ng/mL, 95.3% (41/43) for a PSA of 5 to <10 ng/mL, and 96.8% (30/31) for a PSA of ≥10 ng/mL (Fig. 2). The detection rates were significantly different according to the 3 different PSA ranges (P = 0.0377). Figure 3 demonstrates the number and percentage of all patients with findings suggestive of recurrent PC separated by different locations. PSA was significantly higher in patients with positive 68Ga-PSMA ligand PET/CT findings than in patients with negative results (P = 0.0152; Table 2).
Detection efficacy of 68Ga-PSMA ligand PET/CT in relation to ADT (A), PSA (B), type of RT (C), and primary histologic differentiation (D).
Distribution of 68Ga-PSMA ligand PET/CT findings suggestive of recurrent PC.
PSA Values in Study Population Considering 68Ga-PSMA Ligand PET/CT Results, ADT, GS, and Type of RT
Effect of ADT, GS, and Type of RT
Detection efficacy was significantly higher in patients with ADT compared with patients without ADT (P = 0.0381; Fig. 2). Suggestive lesions were detected in 97.7% (44/45) of patients with ADT and 86.3% (63/73) of patients without ADT. PSA values between both patient groups were not significantly different (P = 0.087; Table 2).
Considering the histopathologic differentiation of the primary PC, 68Ga-PSMA ligand PET/CT showed positive findings in 90.2% (54/61) of patients with a GS ≤ 7 and in 92.0% (23/25) of patients with a GS ≥ 8 (P = 0.635; Fig. 2). Again, PSA values between both patient groups were not significantly different (P = 0.056; Table 2).
There was a significantly higher detection rate with respect to the type of primary RT, which was 97.4% (75/77) in patients after EBRT and 78.0% (32/41) in patients after brachytherapy (P = 0.0006; Fig. 2). PSA values in these patient groups did not differ significantly (P = 0.340; Table 2). However, a significantly higher portion of the patients with EBRT had ADT compared with those with brachytherapy (49.4% vs. 17.1%; P = 0.0006). Regarding the type of RT, the detection rates did not differ significantly between patients with and without ADT in this subgroup analysis (Supplemental Fig. 1).
Influence of Clinical and Pathologic Features on SUVs
The univariate linear regression analyses showed a significant correlation between PSA values, GS, ADT, and SUVs (all P < 0.04; Supplemental Table 1). In a first multivariable linear regression analyses containing PSA, iPSA, GS, ADT, type of RT, acquisition time, injected activity, age, and body mass index, the overall model was significantly superior to a null model without covariates (P = 0.024 and 0.030 with respect to SUVmax and SUVmean as dependent variables, respectively), but no variable was significantly independently associated with SUV (the analysis was limited to n = 67 because of missing data for GS and iPSA; Supplemental Table 2). A second multivariable linear regression analysis (excluding GS and iPSA data to include more patients; n = 107) revealed a significant association of PSA and ADT with SUVs (Table 3).
Multivariable Linear Regression Analyses: Influence of Clinical and Pathologic Features on SUVs
Histopathology and Follow-up
In 6 patients, 68Ga-PSMA ligand PET/CT positive local recurrence or metastases were histologically confirmed (Fig. 4). In 29 patients, follow-up imaging (PET/CT, PET/MRI, bone scintigraphy, CT) indisputably proved that the positive findings were metastases or local recurrence of PC. In another 3 patients, RT or chemotherapy followed by a substantial decrease in PSA or decreasing PSMA uptake of suspicious findings in a consecutive 68Ga-PSMA ligand PET/CT scan proved the malignant nature of PSMA-positive lesions (Supplemental Fig. 2).
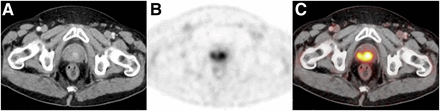
Multifocal local recurrence of PC in a 65-y-old patient (GS, 9; PSA nadir, 0.01 ng/mL after EBRT; staging PSA level, 3.8 ng/mL). CT (A) was negative, whereas PET (B) and fused PET/CT images (C) revealed multiple 68Ga-PSMA ligand–positive lesions in prostate gland (SUVmax, 11.3). This finding was confirmed by transrectal ultrasonography–guided sextant biopsy.
DISCUSSION
To the best of our knowledge, this is the first study investigating the detection efficacy of 68Ga-PSMA ligand PET/CT in a collective of patients with BCR according to the Phoenix criteria after curative intended RT. An overall detection rate of 90.7% indicates that 68Ga-PSMA ligand PET/CT is highly effective in this preselected patient group. A significantly higher detection efficacy (at relatively identical PSA values) as well as a significantly higher SUV for patients with versus without ADT indicate no need for withdrawal of hormonal treatment as discussed for choline-labeled derivates and highlight the potential of possible improved targeting.
BCR after primary curative intended RT is relatively frequent and ranges from 10% to 60% and 7% to 35% in EBRT and brachytherapy, respectively (1,3,4). The aim in these patients is 2-fold: to determine the presence or absence of recurrent disease, and to determine its exact locations, because the disease can be local (25%–30% of cases), systemic (20%–25% of cases), or both (45%–55% of cases) (18). Because of the strong limitations of conventional imaging techniques (CT, MRI, and bone scintigraphy) in detecting the site or sites of relapse, none of the main international guidelines recommend these imaging procedures for patients with biochemical failure after RT, unless the PSA values are markedly elevated (e.g., PSA > 10 ng/mL) or patients are symptomatic (e.g., pain, fracture) (7). According to our results, 68Ga-PSMA ligand PET/CT may offer the possibility in detecting recurrent PC at a clearly earlier time point with the necessary accuracy, which is crucial for further disease management. As an important finding, 68Ga-PSMA ligand PET/CT showed positive findings outside the prostate in 59.8% of patients. Comparable results (i.e., 62.6%) are reported by Ceci et al. for 11C-choline PET/CT (8). Unifocal or multifocal local recurrence by means of 68Ga-PSMA ligand PET/CT was present in 63.5% of patients, which is similar to the findings demonstrated by Ceci et al. and Breeuwsma et al. (62.6% and 71.9%, respectively) (8,19). Notably, the detection or exclusion of local recurrence after primary RT and the finding of metastatic disease not amenable for surgical resection are crucial in view of a potential radical salvage prostatectomy in carefully selected patients (PSA < 10 ng/mL, PSA doubling time > 12 mo, low-dose brachytherapy, GS < 7; according to the guidelines of the European Association of Urology (7)). In addition, precise localization of a limited number of systemic lesions can further advance the increasingly popular concept of treating oligometastatic disease by stereotactic RT.
To date, transrectal ultrasonography–guided biopsy is the current reference standard for the detection of local recurrence in patients with BCR after primary RT. However, it is invasive and may fail to depict some tumors because only a small fraction of the prostate gland is sampled. 68Ga-PSMA ligand PET as a noninvasive promising alternative enabling the assessment of the entire gland could be preferable, as it has already shown promising results for primary PC in combination with MRI (20). Furthermore, with regard to the high detection rates of 68Ga-PSMA ligand PET/CT within the prostate and exact localization of extraprostatic disease (frequently lymph node and bone metastases and even uncommon metastatic manifestations; Fig. 5), more personalized and tailored therapy approaches may be achieved. In particular, the detection of local recurrence together with pelvic lymph node metastases (overall 14 cases in our study) may modify the surgical regimen of intended salvage prostatectomy by adding and guiding lymph node dissection, which has been recently shown (15). Further studies are warranted to evaluate the role of 68Ga-PSMA ligand PET/CT in the therapeutic management of recurrent PC after RT.
An 81-y-old patient with recurrent PC (GS, 8; PSA nadir, 0.5 ng/mL after EBRT; staging PSA level, 3.34 ng/mL). CT images (A) reveal no suspicious finding in penis. Corresponding PET (C) and fused PET/CT images (D) demonstrate high focal uptake (SUVmax, 11.4) in proximal part of penis, indicating soft-tissue metastasis (red arrow). Maximum-intensity projection of whole body (B) shows this penis metastasis and indicates in addition multifocal local recurrence (pink arrow), supra- and infradiaphragmatic lymph node metastases (blue stars), and pelvic bone metastases (green arrows).
In parallel to other PET tracers and reports, our data show an increase in detection rate of 68Ga-PSMA ligand PET/CT with rising PSA values (8,21). To our knowledge, only 3 prior reports, involving 46, 70, and 140 patients, respectively, have investigated the value of 11C- or 18F-labeled choline PET/CT imaging in detecting recurrent PC after EBRT or brachytherapy (8,19,21). The detection efficacy of 90.7% for 68Ga-PSMA ligand PET/CT in our patient cohort is slightly higher than those in the before-mentioned studies, ranging between 80.4% and 87.8%. However, with respect to the results of Breeuwsma et al. (19), the median PSA in our patient population was lower (median PSA, 6.4 ng/mL [range, 2.2–158.4 ng/mL; interquartile range, 4.2–10.2 ng/mL] in our cohort vs. 10.7 ng/mL [range, 0.6–54.7 ng/mL; interquartile range not reported], respectively), indicating a less advanced disease stage in direct comparison and clearly emphasizing the strength of 68Ga-PSMA ligand PET/CT in potentially detecting recurrent PC at an earlier time point of BCR. By contrast, the other 2 studies, by Ceci et al. (8) and Chondrogiannis et al. (21), had lower PSA values with regard to median PSA or PSA range (median PSA, 5 ng/mL, and range, 2–60 ng/mL, in the study of Ceci et al. and range, 1.1–49.4 ng/mL [median PSA not reported], in the study of Chondrogiannis et al., respectively). Nevertheless, our study demonstrates substantial detection efficacies for 68Ga-PSMA ligand PET/CT after primary RT, which is in the range of previous studies (68%–89%) reported for patients with BCR who had been predominantly treated with radical prostatectomy (10,13,22).
Our data show higher detection rates and SUVs (SUVmax/mean as a potential biomarker of PSMA expression) in patients with ADT versus patients without ADT, confirming histologic and immunohistologic reports stating a higher PSMA expression of PC cells in the setting of ADT (23,24). Notably, relatively comparable PSA values could be observed between these patient groups, excluding mere differences due to higher tumor burden. Therefore, it can be concluded that unless PSA values are not considerably suppressed, an ongoing ADT or a new onset ADT shortly before 68Ga-PSMA ligand PET/CT seems not to relevantly reduce diagnostic capability.
Besides ADT, statistically significant associations between PSA and SUVs were shown according to univariate and multivariable linear regression analyses, which could potentially reflect disease activity. For GS, significant correlations to SUVs were detected in the univariate linear regression model, which were not present in the multivariable regression analysis. A positive correlation between increasing GS and PSMA expression is in line with preclinical studies (25,26). Moreover, in a recently published large clinical retrospective study, 68Ga-PSMA ligand PET/CT demonstrated a significantly higher detection efficacy in the setting of GS ≥ 8 in patients with relapsing PC (13), which was attributed to a higher PSMA expression in higher GS.
Interestingly, our data indicate a significantly higher detection rate in patients with EBRT compared with brachytherapy (97.4% vs. 78.0%; P = 0.0006). However, a significantly higher proportion of patients who were treated with EBRT compared with brachytherapy received ADT within 6 mo before imaging. Thus, no clear statement on the efficacy of 68Ga-PSMA ligand PET/CT for EBRT versus brachytherapy can be drawn from these data, because ADT represents a considerable confounding factor according to the results of the multivariable regression analyses in this study.
To date, the Phoenix criteria are the current standard of reference for the definition of BCR after primary RT (2). Although these criteria are highly specific to identify PC relapse, they lack in sensitivity (2), because a prostate gland treated with RT may still harbor relevant disease requiring further treatment without yet fulfilling the Phoenix criteria. In such cases, deferred therapy due to application of current Phoenix criteria may result in worse oncologic or functional outcomes due to local or distant disease progression. Recently, Meeks et al. showed persistent PC after primary RT in 45% of patients submitted to radical cystoprostatectomy, which was performed for bladder cancer at a later time point. Besides, PC was found in 37% of patients without evidence of BCR (27), suggesting that many persistent or recurrent PCs may not meet the Phoenix criteria for intervention. Thus, promising biomarkers other than PSA such as PSMA should be further evaluated to potentially better identify those with viable PC at an earlier time point after RT. Considering the powerful detection efficacy of 68Ga-PSMA ligand PET/CT in our study and the significant association of early salvage treatment at low PSA values with improved biochemical free survival in PC patients after RT (28), PSMA imaging may be performed early in the course of recurrent PC, even if the Phoenix criteria are not fulfilled. However, further studies are needed to evaluate the potential role of 68Ga-PSMA ligand PET/CT at lower PSA values after RT, additionally reconsidering the validity of the Phoenix criteria for detecting PC relapse.
Our study has some limitations. Because it was a retrospective single-institution study, our results may not be generalizable, because imaging acquisitions and interpretation expertise vary across institutions. Despite being retrospective in nature, the particular strength of our study consists in the patient selection strictly including patients with biochemical failure after primary RT as defined by the Phoenix criteria. Next, we did not evaluate the influence of PSA kinetics (velocity and doubling time) on 68Ga-PSMA ligand PET/CT detection rates. We tried to request the series of PSA values needed for these calculations, but nevertheless comprehensive data were missing in more than 80% of patients. However, it has been recently shown that 68Ga-PSMA ligand PET/CT detection rates are not substantially influenced by PSA kinetics (13). Finally, histopathology in each patient would have been preferable but was not feasible for practical and ethical reasons.
CONCLUSION
68Ga-PSMA ligand PET/CT demonstrates a high (>90%) detection efficacy in patients with BCR after primary RT according to Phoenix criteria. The detection rate is dependent on the PSA value and enhanced by ADT. The higher detection rate in patients receiving ADT as well as higher SUVs are compatible with PSMA upregulation during ADT and indicate that unless PSA values are not considerably suppressed, the withdrawal of ADT before 68Ga-PSMA ligand PET/CT is not necessary. ADT could possibly enhance the diagnostic potential by means of target upregulation. 68Ga-PSMA ligand PET/CT can have a crucial impact on further clinical management after BCR in RT-treated patients.
DISCLOSURE
Markus Schwaiger has received funding from the European Union Seventh Framework Program (FP7) under grant agreement no. 294582 ERC grant MUMI. The development of 68Ga-PSMA HBED-CC synthesis was supported by SFB 824 (DFG Sonderforschungsbereich 824, Project Z1) from the Deutsche Forschungsgemeinschaft, Bonn, Germany. The research leading to these results has received funding from the European Union Seventh Framework Program (FP7) under grant agreement no. 256984 EndoTOFPET. No other potential conflict of interest relevant to this article was reported.
Footnotes
Published online Feb. 16, 2017.
- © 2017 by the Society of Nuclear Medicine and Molecular Imaging.
REFERENCES
- Received for publication September 20, 2016.
- Accepted for publication January 24, 2017.