Abstract
We prospectively evaluated and compared the diagnostic performance of 99mTc-hydroxyethylene-diphosphonate (99mTc-HDP) planar bone scintigraphy (pBS), 99mTc-HDP SPECT/CT, 18F-NaF PET/CT, and 18F-NaF PET/MRI for the detection of bone metastases. Methods: One hundred seventeen patients with histologically proven malignancy referred for clinical pBS were prospectively enrolled. pBS and whole-body SPECT/CT were performed followed by 18F-NaF PET/CT within 9 d. 18F-NaF PET/MRI was also performed in 46 patients. Results: Bone metastases were confirmed in 16 patients and excluded in 101, which was lower than expected. The number of equivocal scans was significantly higher for pBS than for SPECT/CT and PET/CT (18 vs. 5 and 6, respectively; P = 0.004 and 0.01, respectively). When equivocal readings were excluded, no statistically significant difference in sensitivity, specificity, positive predictive value, negative predictive value, or overall accuracy were found when comparing the different imaging techniques. In the per-patient analysis, equivocal scans were either assumed positive for metastases (“pessimistic analysis”) or assumed negative for metastases (“optimistic analysis”). The percentages of misdiagnosed patients for the pessimistic analysis were 21%, 15%, 9%, and 7% for pBS, SPECT/CT, PET/CT, and PET/MRI, respectively. Corresponding figures for the optimistic analysis were 9%, 12%, 5%, and 7%. In those patients identified as having bone metastases according to the reference standard, SPECT/CT, 18F-NaF PET/CT, and PET/MRI detected additional lesions compared with pBS in 31%, 63%, and 71%, respectively. Conclusion: 18F-NaF PET/CT and whole-body SPECT/CT resulted in a significant reduction of equivocal readings compared with pBS, which implies an improved diagnostic confidence. However, the clinical benefit of using, for example, 18F-NaF PET/CT or PET/MRI as compared with SPECT/CT and pBS in this patient population with a relatively low prevalence of bone metastases (14%) is likely limited. This conclusion is influenced by the low prevalence of patients with osseous metastases. There may well be significant differences in the sensitivity of SPECT/CT, PET/CT, and PET/MRI compared with pBS, but a larger patient population or a patient population with a higher prevalence of bone metastases would have to be studied to demonstrate this.
See an invited perspective on this article on page 1776.
Bone metastases are frequent in advanced cancers, especially in patients with breast or prostate cancer, and the presence of bone metastases often implies a change of treatment (1,2) and indicates shortened patient survival. Conventional planar bone scintigraphy (pBS) with 99mTc-labeled radiopharmaceuticals, such as hydroxyethylene-diphosphonate (99mTc-HDP), is still the most frequently used modality for diagnosing bone metastases (3). Studies have shown that adding SPECT/CT to pBS improves the specificity and positive predictive value (PPV) as well as the diagnostic confidence of the reader, thereby reducing the number of equivocal study reports (4,5).
18F-sodium fluoride (18F-NaF) was introduced in 1962 by Blau et al. (6). Low affinity to protein, rapid clearance from the plasma, and a first-pass extraction to bone approaching 100% make 18F-NaF an excellent bone-imaging agent (7). In the 1970s, 18F-NaF was replaced by 99Tc-labeled diphosphonate compounds with physical characteristics more suitable for conventional γ-cameras (8).
The more widespread availability of PET/CT scanners and cyclotrons and a more recent global shortage of 99Mo/99mTc generators in the late 2000s initiated a renewed interest for 18F-NaF as an alternative to pBS. Furthermore, 18F-NaF PET is more time efficient for the patient: although pBS is performed after a 2- to 5-h uptake time (9), high-quality PET/CT images can be obtained as soon as 30–45 min after administration of 18F-NaF (10).
Recent metaanalyses have indicated that 18F-NaF PET/CT is more accurate than pBS but the question of whether there is an incremental diagnostic improvement on a patient basis with 18F-NaF PET or PET/CT for bone metastases is not settled (11–13).
The Centers for Medicare & Medicaid Services has covered 18F-NaF PET under the coverage with “an evidence development process” since 2010. In their final decision (Decision Memo for PET (NaF-18) to Identify Bone Metastasis of Cancer (CAG-00065R2), December 2015), after reviewing the last 5 y worth of data, they concluded that there is still not enough evidence to support coverage of 18F-NaF PET to identify bone metastases. The Society of Nuclear Medicine and Molecular Imaging, American College of Nuclear Medicine, and American College of Radiology have pointed out that they strongly disagree with this conclusion.
Meanwhile, whole-body MRI has emerged as an alternative method to detect bone metastases. MRI is more sensitive at detecting early bone marrow lesions than CT. Comparative studies have indicated that MRI is more sensitive and specific than pBS (14–17).
We wanted to test the hypothesis that there is an improved diagnostic performance of 18F-NaF PET/CT compared with conventional pBS and SPECT/CT for the detection of bone metastases on a per-patient basis by conducting, to this date, the largest prospective study on this topic. We also wanted to investigate if there is an added value of combining 18F-NaF PET with MRI using a combined PET/MRI scanner.
MATERIALS AND METHODS
Patients
This study was performed as a prospective clinical study, approved by the local ethics committee (H-4-2012-024). Written, informed consent was obtained from all patients. The inclusion criteria were patients with histologically proven malignancy referred for pBS under the clinical suspicion of bone metastases; and patients able to undergo 18F-NaF PET/CT within 9 d, which was considered justifiable to minimize disparity between scans due to potential progression. Exclusion criteria were known or history of bone metastases, age younger than 18, and pregnant or lactating women. When no contraindications to MRI were identified and there was an available timeslot, whole-body 18F-NaF PET/MRI was performed on the same day as PET/CT in the first 50 patients.
pBS and SPECT/CT
Standard pBS followed by whole-body 99mTc-HDP SPECT/CT were acquired in one session using a hybrid SPECT/CT (Symbia [Siemens Medical Solutions] or Precedence [Philips]) consisting of a dual-head, variable-angle γ-camera combined with a 16-slice helical CT scanner. Anterior and posterior views covering the whole skeleton with the patient supine were obtained 3 h after injection of 99mTc-HDP (mean activity, 586 ± 27 MBq; range, 523–655 MBq; low-energy high-resolution collimators, 10 cm/min). The approximate scan time was 20–25 min.
Whole-body SPECT/CT was performed, without repositioning of the patient, using a whole-body SPECT software option covering 3 bed positions from the tip of the head to the mid thighs. SPECT, low-dose CT, and reconstructions parameters are specified in Table 1. The approximate scan time was 30–35 min.
Scanning and Reconstruction Specifications
PET/CT
Whole-body PET/CT from head to toe with the patient supine was performed on either a 128-slice or 64-slice Biograph mCT or a 40-slice Biograph TrueV scanner (Siemens Medical Solutions). The scan was obtained 45 min (mean, 49 ± 10 min; range, 30–83 min) after injection of 18F-NaF (mean activity, 210 ± 13 MBq; range, 151–239 MBq). The approximate scan time was 30–35 min. A rough estimation of the effective dose from the low-dose CT component was made on the basis of dose–length product and conversion factors described in International Commission on Radiological Protection publication 102 (18).
PET/MRI
Simultaneous PET/MRI from tip of the head to mid thigh was performed after completion of the PET/CT on a 3-T Biograph mMR scanner (Siemens Medical Solutions) using a head and neck coil and 4 body surface coils. The mean time from injection to scan was 124 ± 23 min (range, 89–181 min).
Attenuation correction was performed using a Siemens standard 4-compartment-attenuation map. Noncontrast sequences for all bed positions (in most cases 5) included coronal whole-body T1 turbo spin echo (repetition time/echo time [TR/TE], 600/8.7 ms; flip angle, 140°; slice thickness/gap, 5/1 mm; matrix, 186 × 384; in-plane resolution, 1.25 × 1.25 mm2; scan time, 1:25–3:12 min/bed), coronal whole-body short tau inversion recovery (TR/TE, 5,000/84 ms; flip angle, 125°; slice thickness/gap, 5/1.5 mm; matrix, 186 × 384; in-plane resolution, 1.17 × 1.17 mm2; scan time, 1:47–2:50 min/bed), and sagittal short tau inversion recovery covering the spine (TR/TE, 2,110/8.6 ms; flip angle, 150°; slice thickness/gap, 3/0.6 mm; matrix, 224 × 320; in-plane resolution, 1.3 × 0.9 mm2; scan time, 2:28–5:14 min/bed). In addition, sagittal T1 turbo spin echo also covering the spine (TR/TE, 600/9.1 ms; flip angle, 150°; slice thickness/gap, 3.0/0.6 mm; matrix, 288 × 384; in-plane resolution, 1.0 × 1.0 mm2; scan time, 1:03 min/bed) was acquired subsequent to PET at 3 bed positions. The sequence scan time could vary with bed position due to variations in number of slices needed for patient coverage and prolongation due to restrictions on specific absorption rate of radiofrequency radiation. The approximate scan time was 60–65 min.
Image Interpretation
All examinations were read on standard workstations. pBS and SPECT/CT were interpreted separately by 2 experienced nuclear medicine specialists with the assistance of a radiologist, and discrepancies were solved in consensus. 18F-NaF PET/CT were read by 2 other experienced nuclear medicine specialists with the assistance of 2 radiologists, and discrepancies were solved in consensus with a third nuclear medicine specialist. 18F-NaF PET/MRI were read by a nuclear medicine specialist together with a MR radiologist specialized in musculoskeletal MRI. All readers were masked to the other imaging modalities. Each scan was categorized on a per-patient basis as bone metastases present, widespread metastases (>20 bone metastases present), benign (bone metastases absent), or equivocal. On the basis of these data, 3 analyses were performed: first, excluding all equivocal readings—consensus reading; second, categorizing equivocal readings as benign—optimistic analysis; and third, categorizing equivocal readings as suggestive of malignancy—pessimistic analysis (15,19).
Reference Standard
Results from the interpretations were held up against final diagnoses as confirmed by histologic evaluation, clinical follow-up, or other imaging studies. At least 6-mo clinical follow-up, including review of all regional hospitals’ medical records, biopsies, laboratory reports, and all subsequent imaging, was used. The progression of index lesion on subsequent imaging or lytic lesion changing to blastic lesion during treatment, but also typical appearance of multifocal disease and increased lesion number over time, was strong evidence of bone metastases. Scans were considered false-negative if follow-up revealed bone metastases within 6 mo.
Statistical Analysis
The sample size calculation was prospectively determined to be 120 (power of 80%, α of 5%) to detect a difference of 13%, as we estimated that approximately 30% of patients would have bone metastases.
Data analysis was performed using SPSS (version 19; IBM Corp.). A patient-based data analysis was performed. Sensitivity, specificity, accuracy, PPV, and negative predictive value for pBS, SPECT/CT, PET/CT, and PET/MRI were compared using the McNemar test. Two-sided P values were calculated, and a P value of less than 0.05 was considered statistically significant. Frequency of equivocal readings was compared with the χ2 test.
RESULTS
Between June 2012 and January 2015, 488 patients referred for a clinical pBS met the inclusion criteria and were invited to participate. Figure 1 shows patient inclusion leading to a total of 117 patients available for study evaluation: 62 men with prostate cancer, 54 women with breast cancer, and 1 woman with renal cancer. Patient characteristics are listed in Table 2, and reasons for patient referral are listed in Table 3.
Flowchart illustrating patient inclusion and exclusion criteria.
Patient Characteristics
Clinical Indication for Requesting Bone Scanning Overall and Stratified by Cancer Type
The 18F-NaF PET/CT was performed 5.3 ± 2.3 d after pBS and SPECT/CT. Forty-six patients underwent a supplemental 18F-NaF PET/MRI (on the same day as the PET/CT). The approximate effective dose was 4 mSv from the pBS and an additional 4–5 mSv from the low-dose CT incorporated in the SPECT/CT examination. The effective dose from 18F-NaF PET/CT was approximately 6 mSv from the tracer and an additional 4–5 mSv from the low-dose CT, resulting in a total dose of 10–11 mSv. The PET/MRI did not cause any additional radiation dose to the patient.
Follow-up
The average follow-up period was 652 ± 217 d (range, 130–1,090). Bone metastases were confirmed in 16 patients (14%) (4 breast cancer patients and 12 prostate cancer patients) based on the reference standard (Table 4). Bone metastases were excluded in the remaining 101 patients (86%). Four patients died during follow-up, 130, 140, 669, and 705 d after the initial pBS. Sixty-six patients (56%) underwent relevant imaging within the follow-up period and 45 (38%) within 6 mo.
Patients with Confirmed Bone Metastases Based on Reference Standard
Patient-Based Analysis
Ninety-one of 117 (true-positive and true-negative cases) patients were correctly diagnosed with pBS including 9 of 16 with bone metastases and 82 of 101 without bone metastases. Eighteen pBS readings were equivocal (2 with bone metastases and 16 without) (Table 5). Ninety-nine of 117 patients were correctly diagnosed with SPECT/CT including 9 of 16 with bone metastases and 90 of 101 without. Five SPECT/CT readings were equivocal (1 with bone metastases and 4 without). One hundred two of 117 patients were correctly diagnosed with PET/CT including 12 of 16 with bone metastases and 94 of 101 without. Six PET/CT readings were equivocal (1 with bone metastases and 5 without). Forty-three of 46 patients were correctly diagnosed with PET/MRI including 6 of 7 with bone metastases and 37 of 39 without. No PET/MRI readings were equivocal. All modalities missed the same 2 patients diagnosed with bone metastases 4.5 and 5 mo after the initial pBS.
Patient-Based Analysis
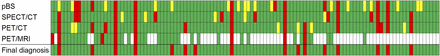
The number of equivocal pBS scans, 18, was significantly higher than for SPECT/CT (5 scans, P = 0.004) and PET/CT (6 scans, P = 0.01) (Table 5). None of the 46 PET/MRI scans was classified as equivocal. Figure 2 illustrates interpretations and the final diagnosis for all patients.
All scans with 1 patient in each column. Red = positive scan finding or final diagnosis; green = negative scan finding or final diagnosis; yellow = equivocal scan finding; white = not performed.
When equivocal readings were categorized as malignant (pessimistic analysis), pBS misdiagnosed 24 (21%) patients, SPECT/CT 17 (15%) patients, and PET/CT 10 (9%) patients. The corresponding figures for optimistic analysis were 10 (9%) misdiagnosed by pBS, 14 (12%) by SPECT/CT, and 6 (5%) by PET/CT. PET/MRI misdiagnosed 3 (7%) patients in both cases.
Diagnostic Accuracy
When equivocal readings were excluded, no statistically significant difference in sensitivity, specificity, PPV, negative predictive value, or overall accuracy were found when the different techniques were compared. Table 6 summarizes the diagnostic performance when optimistic analysis and pessimistic analysis are applied.
Diagnostic Accuracy
Imaging Findings
Among the 16 patients with bone metastases, 3 patients were categorized by PET/CT to have widespread metastases (>20 lesions) whereas SPECT/CT showed more than 20 lesions in two of them and pBS only characterized one of them to have widespread bone metastases. Compared with pBS, SPECT/CT showed additional lesions in 5 of these 16 patients (31%) and PET/CT in 10 (63%). Seven of the 16 patients with bone metastases underwent PET/MRI. PET/MRI revealed additional lesions in 5 of 7 of these patients (71%) compared with pBS, and in 2 (28%) of them also lesions not identified on SPECT/CT and PET/CT (example in Fig. 3 and Supplemental Fig. 1 [supplemental materials are available at http://jnm.snmjournals.org). In 2 cases, PET/MRI indicated in contradiction to the other modalities presence of single bone metastases but in both cases follow-up could not verify the metastatic lesions.
A 51-y-old woman treated for locally advanced breast cancer. (A and B) pBS; anterior and posterior views. (C and D) SPECT/CT; maximum-intensity-projection (MIP) and fused image. (E and F) PET/CT; MIP and fused image. (G–I) PET/MR; MIP, fused, and T1 turbo spin echo image. All modalities identified uptake in left rib and in left femoral neck (solid arrows). pBS was interpreted as equivocal. SPECT/CT, PET/CT, and PET/MRI were all interpreted as positive for bone metastases based on lesion in left hip. Lesion in rib represented fracture on CT. PET/CT and PET/MRI identified additional lesions; 2 are marked with hollow arrows. More details and follow-up images are provided in Supplemental Figure 1.
DISCUSSION
To our knowledge, this study is the largest prospective study on the diagnostic performance of 18F-NaF PET/CT compared with conventional pBS and SPECT/CT for the detection of bone metastases. It is also the first to include 18F-NaF PET/MRI.
In the studied patient population, SPECT/CT, PET/CT, and PET/MR detected additional lesions in a relatively high percentage of those patients identified as having bone metastases, 31%, 63%, and 71%, respectively. But on a patient level, despite the technologic advantages of SPECT/CT, PET/CT, and PET/MR, they correctly changed only the tumor stage in a relatively small fraction of patients compared with pBS. The percentage of misdiagnosed patient for the optimistic analysis were 9%, 12%, 5%, and 7% for pBS, SPECT/CT, 18F-NaF PET/CT, and 18F-NaF PET/MR, respectively.
These results are not completely in line with the last 2 decades of studies on diagnostic accuracy of 18F-NaF PET, summarized in several metaanalyses (11–13). In 2013, Palmedo et al. published a large study comparing whole-body SPECT/CT and pBS in 308 patients with either prostate or breast cancer (5). There was no significant difference in per-patient sensitivity, which was 93%, 94%, and 97% for pBS, SPECT, and SPECT/CT, respectively. Specificity was, on the other hand, significantly better with SPECT/CT. These results are contradictive to prior metaanalyses in which pooled sensitivity for pBS was as low as 47% but more in line with our results.
The lack of significance in our study could be partly explained by the relatively low prevalence of patients with osseous metastases in the study population. The observed prevalence of 14% is lower than expected from our clinical practice and does imply that even after inclusion of 117 patients, we have to conclude that this study was underpowered.
A possible reason for this low prevalence of osseous metastases could be that patients with severe pain, caused by widespread metastatic disease, would be less willing to participate in a study in which they should undergo multiple scans on different days. On the other hand, in patients with widespread metastatic disease it seems even less likely to find a significant difference in sensitivity on patient-based analysis.
The standard of reference was clinical follow-up, including other imaging examinations and histologic evaluation. In our study, histology evaluations were performed on 2 patients. The definition of false-negative scans could be debatable especially if all modalities are negative. What acceptable and reasonable time period should pass after an initial negative scan, before you should consider a scan on a patient, later being diagnosed with bone metastases, as being false-negative? We chose 6 mo, which in some sense could be considered a long time but is a relevant time period in relation to normal imaging frequency.
The nature and verification of small lesions detected by 18F-NaF PET is cumbersome; therefore, follow-up including repetitive imaging is essential if the true diagnostic performance is going to be established. We believe this lack of reference standard in many studies is a weakness, especially in lesion-based analysis. To verify all lesions by obtaining histologic proof is of course impractical and unethical. In lesion-based analysis, the number of lesions included in each patient must also be limited because patients with many true-positive lesions detected only on PET/CT will have too strong an influence on the result. As a consequence of this bias, the problem with verification and the fact that the exact number of bone metastases is of negligible clinical importance, we chose to perform patient-based analysis in our study.
18F-NaF PET/MRI combines 2 methods highly sensitive for changes in, respectively, bone and bone marrow. Thus, we expected an increased diagnostic sensitivity of PET/MRI compared with standard imaging in this setting. However even though PET/MRI in 2 patients could reveal additional lesions not seen with either SPECT/CT or 18F-NaF PET/CT, we could not demonstrate any significant improved diagnostic performance for PET/MRI in our subgroup of 46 patients. Thus, routine diagnosis of bone metastases by 18F-NaF PET/MRI is not likely to prove cost-effective. Instead, future development of the different imaging modalities will influence the modality of choice: the newly introduced improved image reconstructions for bone on SPECT/CT by integrating CT data in the SPECT image reconstruction (20,21); the promising results with new PET tracers, especially 68Ga-PSMA, for imaging both osseous and nonosseous prostate cancer metastases (22); and the continuing development of faster and optimized whole-body MR imaging sequences.
CONCLUSION
In this prospective study, designed to reflect the patient population that routinely undergoes bone scans at our institution, the clinical benefit of using 18F-NaF PET/CT or PET/MR is likely limited. However, this conclusion is influenced by the lower than expected prevalence of patients with osseous metastases. There may well be significant differences in the sensitivity of SPECT/CT, PET/CT, PET/MR, and pBS, but a larger patient population or a patient population with a higher prevalence of bone metastases would have to be studied to confirm or disprove this. 18F-NaF PET/CT and SPECT/CT, however, produces a significantly lower number of equivocal readings than pBS, most likely because of the structural information available with corresponding CT. No significantly improved diagnostic performance was found in the subgroup of 46 patients with PET/MRI.
DISCLOSURE
No potential conflict of interest relevant to this article was reported.
Acknowledgments
We thank Karin Stahr, Marianne Federspiel, Jakup Poulsen, Elisabeth Abrahamnsson, Tim Lundby, M.C.H. Albers, and M.H.B. Frederiksen for all their work in conjunction with scanning of the patients and John and Birthe Meyer foundation for donation of the PET/MRI system.
Footnotes
Published online Aug. 10, 2017.
- © 2017 by the Society of Nuclear Medicine and Molecular Imaging.
REFERENCES
- Received for publication December 26, 2016.
- Accepted for publication July 11, 2017.