Abstract
In newly diagnosed diffuse large B-cell lymphoma (DLBCL), the sensitivity of bone marrow biopsy (BMB) for the detection of bone marrow involvement (BMI) can be low because of sampling error if the BMI is focal and not diffuse. Although 18F-FDG PET/CT is now recommended for initial staging of DLBCL, its role regarding BMI is not well defined. This study evaluated whether 18F-FDG PET/CT, in comparison with BMB, is useful for the detection of BMI and predictive of outcome. Methods: From the 142 patients who were referred to our institution for newly diagnosed DLBCL from June 2006 to October 2011, 133 were retrospectively enrolled in our study. All patients underwent whole-body 18F-FDG PET/CT and a BMB from the iliac crest before any treatment. 18F-FDG PET/CT was considered positive for BMI in cases of uni- or multifocal bone marrow 18F-FDG uptake that could not be explained by benign findings on the underlying CT image or history. A final diagnosis of BMI was considered if the BMB was positive or if the positive 18F-FDG PET/CT was confirmed by guided biopsy or targeted MR imaging or in cases of disappearance of focal bone marrow uptake concomitant with the disappearance of uptake in other lymphoma lesions on 18F-FDG PET/CT monitoring. Progression-free survival and overall survival were analyzed using the Cox proportional hazards regression model. Results: Thirty-three patients were considered to have BMI. Of these, 8 were positive according to the BMB and 32 were positive according to 18F-FDG PET/CT. 18F-FDG PET/CT was more sensitive (94% vs. 24%; P < 0.001), showed a higher negative predictive value (98% vs. 80%), and was more accurate (98% vs. 81%) than BMB. Median follow-up was 24 mo (range, 1–67 mo). Twenty-nine patients (22%) experienced recurrence or disease progression during follow-up, and 20 patients died (15%). In multivariate analysis, only the International Prognostic Index and the 18F-FDG PET/CT bone marrow status were independent predictors of progression-free survival (P = 0.005 and 0.02, respectively), whereas only the International Prognostic Index remained an independent predictor of overall survival (P = 0.004). Conclusion: Assessment of BMI with 18F-FDG PET/CT provides better diagnostic performance and prognostic stratification in newly diagnosed DLBCL than does BMB.
Diffuse large B-cell lymphoma (DLBCL) is the most frequent subtype of high-grade non-Hodgkin lymphoma, which is the sixth most common cause of malignant tumor incidence and mortality in Europe and the United States. With a 150% increase in incidence in recent decades, non-Hodgkin lymphoma is a major public health problem (1).
The International Prognostic Index (IPI) was developed to predict prognosis in patients with aggressive non-Hodgkin lymphoma. One point is assigned for each of the following factors: age greater than 60 y, stage III or IV according to the Ann Arbor classification, elevated serum lactate dehydrogenase (LDH), Eastern Cooperative Oncology Group (ECOG) performance status of 2 or more, and more than 1 extranodal site of disease. An IPI score of 1 or less is associated with a 5-y overall survival (OS) higher than 70%, whereas a score of 2 has a 5-y OS of 51% and a score of more than 2 has a 5-y OS lower than 50% (2). Bone marrow involvement (BMI) is diagnosed using trephine bone marrow biopsy (BMB) in approximately 20% of DLBCL and accounts for 1 point in the IPI score (2–5). In this context, accurate staging of bone marrow status is crucial to optimize the therapeutic strategy (5,6). BMB remains the gold standard to determine bone marrow status but has poor sensitivity (∼50%) because the sample size may be small or the BMI focal (7,8). Furthermore, it is an invasive procedure that is not free from the risk of complications such as pain, hemorrhage, or infection (9,10). Another noninvasive diagnostic tool could thus be useful.
Although whole-body 18F-FDG PET/CT is now a routine procedure for the initial staging of DLBCL, its role in bone marrow assessment is not yet well defined (11). Previous studies suggested potential interest in 18F-FDG PET/CT (detection of false-negative BMBs and guidance of the biopsy) for the detection of BMI in DLBCL (12–20). However, information on the prognostic value of BMI revealed by 18F-FDG PET/CT is lacking (20). The objective of our study was thus to evaluate whether 18F-FDG PET/CT, in comparison with BMB, is useful for the detection of BMI and is predictive of outcome.
MATERIALS AND METHODS
Patient Population
One hundred forty-two consecutive patients with a first diagnosis of DLBCL between June 2006 and October 2011 were retrospectively enrolled in this study. The procedures were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2008. All patients signed a written informed consent form.
The inclusion criteria were adult patients with DLBCL diagnosed on biopsy (nodal or extranodal lesion) and for whom the BMB and initial 18F-FDG PET/CT results were available. Exclusion criteria were patients with prior known and treated lymphoma (of high or low grade), those with Richter transformation, those who had received hematopoietic growth factor injections less than 48 h before the first 18F-FDG PET/CT scan, and those for whom more than 60 d had passed between BMB and the first 18F-FDG PET/CT scan (21). Six patients were excluded from the study because of prior known and treated lymphoma or Richter transformation and three because the time between the BMB and 18F-FDG PET/CT scan was more than 60 d.
All patients underwent a diagnostic checkup before beginning the therapy, as follows: physical examination; routine blood parameters including a hemogram, and LDH; CT scan of the neck, chest, abdomen, and pelvis; and BMB and 18F-FDG whole-body PET/CT. The median interval between 18F-FDG PET/CT and BMB was 5 d (first quartile, 2 d; third quartile, 11 d). The IPI score (Ann Arbor stage and extranodal involvement) was determined without considering bone marrow uptake on the 18F-FDG PET/CT study.
The patients received 4 cycles of a combination of rituximab and CHOP chemotherapy (cyclophosphamide, hydroxydaunomycin, vincristine, and prednisone) or a CHOP-like chemotherapy regimen (AVCVBP [doxorubicin, cyclophosphamide, vindesine, bleomycin, and prednisone]) as the induction treatment. The consolidation treatment was determined according to the age of the patients and IPI risk factors at baseline and consisted of either additional cycles of standard immunochemotherapy or, for young patients with a high-risk IPI, intensified chemotherapy followed by autologous stem cell transplantation. The response to treatment was assessed using CT and 18F-FDG PET/CT at the middle and end of treatment.
18F-FDG PET/CT Acquisition
Whole-body PET was performed sequentially using a dedicated PET/CT system (Gemini GXL or Gemini TOF; Philips). CT scans were not of diagnostic quality and were used for anatomic registration and for attenuation correction. Emission data were corrected for dead time, random and scatter coincidences, and attenuation before reconstruction with the row-action maximum-likelihood algorithm iterative method. The image voxel counts were calibrated to activity concentration (Bq/mL) and were decay-corrected using the time of tracer injection as a reference.
All patients were instructed to fast for at least 6 h before the injection of 18F-FDG. Serum glucose levels were measured by the hexokinase method. Whole-body emission and transmission scans were acquired in the 3-dimensional mode, 60 min after the intravenous administration of a 3–5 MBq/kg dose of 18F-FDG. Diagnostic-quality unenhanced CT images were acquired before PET data acquisition. The CT, PET, and coregistered PET/CT images were reviewed in transaxial, coronal, and sagittal planes along with maximum-intensity-projection whole-body images.
BMB
Bone marrow samples were collected by unilateral trephine biopsy of the posterior iliac crest.
Histologic and biomolecular analyses of samples were performed according to standard procedures. Diffuse or nodular lymphoma infiltrates were always compared with morphology in biopsy samples from lymph nodes or other extramedullary sites. BMI was defined according to the 2008 World Health Organization classification of lymphomas (22). Whenever required, immunohistochemical stains were used to define the lineage of lymphoma cells.
The BMB was considered positive in the presence of either large-cell or small-cell lymphoma involvement.
18F-FDG PET/CT Analysis and BMI
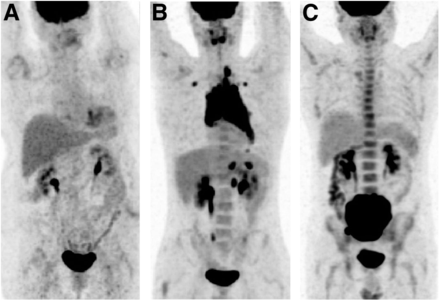
To determine the independent diagnostic and prognostic value of 18F-FDG PET/CT, images were reinterpreted by an experienced reader who was unaware of other clinical and BMB findings. 18F-FDG PET/CT findings were considered positive for BMI in cases of uni- or multifocal bone marrow 18F-FDG uptake that could not be explained by benign findings on the underlying CT image or history (e.g., fractures) (19,23). In contrast, diffuse intense bone marrow 18F-FDG uptake was considered negative for BMI; indeed, it has been demonstrated that diffuse intense uptake is significantly related to anemia or inflammatory processes (Fig. 1) (24–26).
Diffuse bone marrow uptake pattern in 18F-FDG PET/CT. (A and B) Uptake lower than (A) or similar to (B) that in liver was considered negative for BMI. (C) Uptake higher than that in liver was always linked to anemia or inflammatory processes and also considered negative for BMI.
The final diagnosis of BMI was retained in cases of positive pathologic findings (BMB) or positive 18F-FDG PET/CT confirmed by guided biopsy or targeted MR imaging or, after chemotherapy, by the concomitant disappearance of focal bone marrow uptake and uptake in other lymphoma lesions on 18F-FDG PET/CT reassessment (Figs. 2 and 3).
Unifocal bone marrow uptake pattern in 18F-FDG PET/CT. Focal lesion on right pelvic bone (A) was shown to be located on right part of sacrum (B), with no underlying anomaly on CT (C). Usual BMB in left posterior iliac crest was negative. Targeted MR imaging confirmed BMI. According to our criteria, patient was considered to have BMI.
Patient with negative initial BMB of left posterior iliac crest. (A) Initial 18F-FDG PET/CT highlighted multifocal uptake in bone marrow. Guided biopsy of right iliac crest came back positive. (B) 18F-FDG PET/CT monitoring revealed excellent metabolic response. According to our criteria, this patient was considered to have BMI.
In contrast, bone marrow status was considered normal in cases of negative BMB results and diffuse bone marrow 18F-FDG uptake, in cases of positive 18F-FDG PET/CT findings invalidated by guided biopsy or targeted MR imaging, or in cases of persistent focal bone marrow uptake on 18F-FDG PET/CT monitoring despite disappearance of uptake in other lymphoma lesions (15,16,23).
Follow-up
Progress updates were obtained from the medical record. When relevant, details of the date and cause of death were obtained. The disease status at the time of death was recorded. Patients were considered in progression or relapse of DLBCL in cases of positive results on 18F-FDG PET/CT monitoring or if other means detected the disease (biopsy or CT scan).
Statistical Analysis
Continuous data were expressed as mean (±SD), and qualitative data were expressed as numbers and percentages. Patients were categorized according to the absence or presence of BMI according to the BMB and 18F-FDG PET/CT results. The 2 groups were compared using the Student t test for continuous variables and the Fisher exact test for qualitative variables.
Progression-free survival (PFS) was defined as the time from lymphoma diagnosis to the first progression or relapse, or death from any cause, at the last follow-up. OS was defined as the time from lymphoma diagnosis to death from any cause, at the last follow-up.
Kaplan–Meier survival curves with the log-rank test were performed to analyze OS and PFS, according to BMB status and 18F-FDG PET/CT bone marrow status. Univariate and multivariate Cox proportional hazards regression models were performed for IPI factors and score, BMB status, and 18F-FDG PET/CT bone marrow status.
P values less than 0.05 were considered significant.
RESULTS
Patient Characteristics
Of the 142 patients, 133 (male-to-female ratio, 1) were eligible for analysis. The mean age at diagnosis was 57 y (range, 18–87 y), 8 patients (6%) had BMI on BMB, whereas 32 patients (24%) had BMI according to 18F-FDG PET/CT findings. Among patients with BMI detected by BMB, 3 (37.5%) had discordant BMI (small-cell lymphoma), all of whom had BMI according to 18F-FDG PET/CT. Fifty-three patients (39.8%) had an IPI score above 2, 84 (63.2%) had elevated LDH, 99 (74.4%) were stage III or IV according to the Ann Arbor classification, 23 (17.3%) had an ECOG score of 2 or more, and 28 (21%) had more than 1 extranodal site of involvement.
BMI and Baseline Clinical Characteristics
Baseline clinical characteristics according to the absence or presence of BMI (according to BMB and 18F-FDG PET/CT) are shown in Table 1. There were no significant differences between the groups concerning sex, age, LDH, an ECOG score of 2 or more, and IPI score.
Baseline Clinical Characteristics According to Bone Marrow Status by BMB or 18F-FDG PET/CT
Diagnostic Performance
Thirty-three patients had confirmed BMI according to our criteria and were also defined as having BMI by referring physicians. 18F-FDG PET/CT correctly evaluated bone marrow status in 130 cases (accuracy, 97.7%; 95% confidence interval (CI), 97.7% ± 2.5%), whereas the BMB was correct in 111 cases (accuracy, 81.2%; 95% CI, 81.2% ± 6.6%).
18F-FDG PET/CT was negative for BMI in 101 patients. Of these, 99 also had negative BMB results. The 2 remaining patients had BMI according to the biopsy.
18F-FDG PET/CT was reported as positive for BMI in 32 patients because of the presence of focal bone marrow uptake. Only 1 of these was considered false-positive (myeloproliferative syndrome on BMB). Among the 31 other patients, only 6 had a positive BMB. The remaining 25 patients had confirmed BMI according to our criteria (2 by targeted MR imaging, 3 by guided biopsy, and 20 by disappearance of bone marrow uptake concomitant with reduced uptake in other 18F-FDG–avid lymphoma lesions on PET/CT monitoring).
Only 4 patients presented a unifocal pattern of 18F-FDG uptake. None of these foci were in the posterior iliac crest, and none of the patients had a positive BMB. Two of these patients were already considered stage IV, one as stage III, and the other as stage II. In this last patient, targeted MR imaging was also consistent with BMI. Neither guided biopsy nor targeted MR imaging was performed for the other 3 patients.
According to our data, 11 patients experienced diffuse bone marrow 18F-FDG uptake with an intensity higher than in the liver. All had anemia or an inflammatory syndrome that could explain this pattern of uptake. Nine of these patients were considered stage IV and 2 stage III. Only one of them showed BMI on BMB. This patient, with an IPI of 5, was already considered stage IV.
The sensitivity of 18F-FDG PET/CT was significantly higher than that of BMB (93.9% vs. 24.2%, P < 0.001; 95% CI, 93.9% ± 8.1% vs. 24.2% ± 14.6%), as was the negative predictive value ([NPV] 98% vs. 80%; 95% CI, 98% ± 2.7% vs. 80% ± 7%). In contrast, the 2 procedures did not significantly differ in specificity (99% vs. 100%; 95% CI, 99% ± 1.9% vs. 100% ± 0%) or positive predictive value ([PPV] 96.9 vs. 100%; 95% CI, 96.9% ± 6% vs. 100% ± 0%) (Fig. 4).
Diagnostic performance of BMB and 18F-FDG PET/CT regarding BMI (95% CIs).
Impact of 18F-FDG PET/CT Findings on Staging
Among the 26 patients with positive 18F-FDG PET/CT results and negative BMB results, 11 were upstaged to stage IV by PET/CT according to the bone marrow status (1 patient was initially stage I, 3 were stage II, and 7 were stage III). These 4 patients with initial stage I or II benefited from a change in consolidation treatment consisting of additional cycles of standard immunochemotherapy for 2 patients and intensified chemotherapy followed by autologous stem cell transplantation for the other 2. The bone marrow 18F-FDG uptake pattern was unifocal for 2 patients and multifocal for the other 2. All had a confirmed BMI by guided biopsy or targeted MR imaging. None died, and only one experienced recurrence of the disease. Twelve patients had an IPI below 2, and 14 had an IPI of 2 or more.
The only patient with false-positive 18F-FDG PET/CT findings had already been assigned to stage IV because of known extranodal involvement (kidney).
Among the 2 patients with negative 18F-FDG PET/CT findings and positive BMB findings, one had an IPI score of 4 and the other had an IPI score of 5; the two were assigned to stage IV without consideration of bone marrow 18F-FDG uptake.
Survival Analysis
Survival data were analyzed with a closeout date of the first of February 2012. The status of all 133 patients enrolled in this study was known at the closeout date.
With a median follow-up of 24 mo (range, 1–67 mo), 29 patients (21.8%) experienced either progression or relapse of the lymphoma and 20 patients (15%) died. For the overall population, the estimated PFS at 2 y was 79.2% ± 2.7% and the estimated OS at 2 y was 85.5% ± 2.4%.
Two-year OS was higher for patients with a negative BMB than for patients with a positive BMB (87.2% ± 3.4% vs. 62.5% ± 17.1%, respectively; P = 0.007). PFS also differed significantly (P = 0.002) between the 2 groups, with estimates of 2-year PFS at 82.1% ± 3.7% and 37.5% ± 17.1%, respectively (Fig. 5).
PFS according to BMB (A) or 18F-FDG PET/CT status (B).
The same pattern was observed for 18F-FDG PET/CT bone marrow status. Two-year OS was higher for patients without BMI on 18F-FDG PET/CT than for patients with BMI on 18F-FDG PET/CT (88.5% ± 3.7% vs. 76.1% ± 8%, respectively; P = 0.02). PFS also differed significantly (P < 0.001) between the 2 groups, with estimates of 2-y PFS at 84.5% ± 3.9% and 62.5% ± 9.2%, respectively (Fig. 5).
Patients with a negative BMB and positive 18F-FDG PET/CT (26 patients) had worse PFS than patients with a negative BMB and 18F-FDG PET/CT (99 patients, P = 0.006) (Fig. 6).
PFS of patients with negative BMB (A) or positive BMB (B) considering their 18F-FDG PET/CT status.
By univariate analysis, factors predictive of both OS and PFS included an IPI score above 2, BMI according to BMB, and BMI according to 18F-FDG PET/CT (Tables 2 and 3). Regarding IPI factors, age over 60 y and Ann Arbor stage III or IV were not predictive of OS and PFS, whereas an ECOG score of 2 or more, elevated LDH, and more than one extranodal site were predictive (Tables 2 and 3).
Cox Regression Analysis for PFS
Cox regression Analysis for OS
By multivariate analysis, only an IPI score above 2 and BMI detected by 18F-FDG PET/CT remained independent predictive factors of PFS, and an IPI score above 2 alone remained an independent predictive factor of OS (Tables 2 and 3).
DISCUSSION
Currently, only BMB is recommended for the evaluation of BMI in DLBCL (11,27). However, it is not a reliable method since BMI in DLBCL is usually focal and less than 30% of the bone marrow is infiltrated with lymphoma in this case (7,8,28).
Our study suggests, in a series of 133 patients with newly diagnosed DLBCL, that the diagnostic performance of 18F-FDG PET/CT regarding bone marrow status is better than that achieved using the gold standard, BMB. Moreover, bone marrow status according to 18F-FDG PET/CT appears to be a better independent prognostic factor than bone marrow status according to BMB.
Several studies have already described the capacity of 18F-FDG PET/CT to detect BMI in patients with DLBCL (12–19,23). In our homogeneous series of patients, BMI was assessed in 25% of patients according to our criteria. These findings are in agreement with previous studies (2–5). We confirmed that 18F-FDG PET/CT and BMB have similar specificity and PPV. However, 18F-FDG PET/CT was significantly better than BMB for sensitivity, NPV, and accuracy. The sensitivity, NPV, and accuracy of 18F-FDG PET/CT in our series were similar to those previously reported (12,14–16,19,23). Furthermore, after BMI was detected by 18F-FDG PET/CT, 11 patients were upstaged (<10%, compared with 21% in the study of Pelosi et al. (15)). For 4 of these, there was a significant impact on the therapeutic management.
Knowledge about the prognostic value of BMI based on 18F-FDG PET/CT is limited (20). In our study, the prognosis in patients with BMI detected by either BMB or 18F-FDG PET/CT was worse than that in patients without BMI. This result was expected given the strong correlation between Ann Arbor status, IPI score, and bone marrow status (3). Nevertheless, our results suggest that bone marrow status according to 18F-FDG PET/CT is an independent prognostic factor for PFS, whereas bone marrow status according to BMB is not. In addition, in patients with a negative BMB, the bone marrow status assessed by 18F-FDG PET/CT had a significant additional impact on the prognostic stratification.
Unlike most previous studies, which included high-grade non-Hodgkin lymphoma, we decided to evaluate BMI in a homogeneous population of only DLBCL patients (12,14–18). However, patients were enrolled retrospectively and the histologic control of hypermetabolic bone marrow lesions was performed according to the clinician’s decision. Therefore, only a limited number of patients underwent guided biopsy after 18F-FDG PET/CT, and our study may have overestimated BMI based on 18F-FDG PET/CT monitoring alone. Nevertheless, guided biopsy, targeted MR imaging, and 18F-FDG PET/CT have already been used as standard methods in the literature to identify BMI (23). Furthermore, in several studies, all lesions that underwent guided biopsy on the basis of 18F-FDG PET/CT results were positive for BMI (16,17).
18F-FDG PET/CT analyses were performed by a masked, trained observer, and the signs of diffuse or focal uptake were easy to interpret (29).
Although biopsy samples were analyzed by a trained pathologist, only 8 patients had positive results on BMB (<10%). Nevertheless, the incidence of BMI detected by unilateral or bilateral BMB in DLBCL patients is highly variable in the literature (from 9% to 31%) (5,13,15,23).
18F-FDG PET/CT and biopsy were discordant regarding bone marrow status in 28 patients. Of these, 26 had a negative bone marrow status according to the biopsy and a positive status according to 18F-FDG PET/CT; in contrast, 2 patients had a positive bone marrow status according to the biopsy and a negative status according to 18F-FDG PET/CT. The first category included only 1 case of false-positive 18F-FDG PET/CT: BMB revealed a myeloproliferative syndrome, but the patient had already been assigned to stage IV because of known extranodal involvement (kidney). Regarding the second category, no explanation was found since both had large B-cell involvement of the bone marrow and 18F-FDG–avid nodal lesions.
BMI evaluated by either BMB or 18F-FDG PET/CT failed to independently predict OS; however, the relatively small number of deaths during follow-up (20/133 patients) and the inclusion of all causes of death may be a limitation.
Three other studies that used the same criteria to evaluate BMI (BMB plus 18F-FDG PET/CT monitoring plus guided biopsy or MR imaging) in high-grade non-Hodgkin lymphoma reported results comparable to ours regarding the diagnostic performance of 18F-FDG PET/CT (15,16,23). Indeed, overall sensitivity was estimated at 90.4%, specificity at 97.6%, accuracy at 95.4%, NPV at 95.8%, and PPV at 94.3%, compared with 93.9%, 99%, 97.7%, 98%, and 96.9%, respectively, in our study (Tables 4 and 5). Two of these studies also evaluated the diagnostic performance of BMB and reported results similar to ours: 58%, 100%, 88.3%, 86.1%, and 100%, respectively, versus 24.2%, 100%, 81.2%, 80%, and 100%, respectively, in our study (Tables 4 and 5) (15,23).
Correlation Between 18F-FDG PET/CT and BMB Results and Final Bone Marrow Status in Our Study
Correlation Between 18F-FDG PET/CT and BMB Results and Final Bone Marrow Status in 3 Studies
According to these results, a simple algorithm could be suggested to evaluate bone marrow status, since all patients undergo 18F-FDG PET/CT assessment for the initial staging. If 18F-FDG PET/CT is consistent for BMI (according to previously described criteria), either no biopsy is needed (considering the high PPV of PET/CT) or guided biopsy is suggested in order to confirm BMI. If 18F-FDG PET/CT shows no evidence of BMI, either no biopsy is needed (considering the high NPV of PET/CT) or iliac-crest BMB is suggested. In current practice, it remains to be determined whether the risk of underestimating BMI with a bone marrow assessment by 18F-FDG PET/CT alone is clinically acceptable (risk of <3% considering the different studies, 9 patients of 370) and whether the histologic characterization of bone marrow lesions is useful. Further prospective studies that compare the 2 strategies could be useful to validate this approach.
CONCLUSION
In this large, homogeneous population of 133 newly diagnosed DLBCL patients from a single institution, BMI determined by 18F-FDG PET/CT demonstrated a better diagnostic and prognostic performance than did unilateral iliac-crest biopsy. In addition, the bone marrow status assessed by 18F-FDG PET/CT is an independent predictor of PFS, emphasizing the crucial role of 18F-FDG PET/CT in the initial staging of DLBCL. Thus, 18F-FDG PET/CT may replace BMB for baseline bone marrow assessment in DLBCL patients or at least may be considered a complementary tool in this setting.
DISCLOSURE
The costs of publication of this article were defrayed in part by the payment of page charges. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734. No potential conflict of interest relevant to this article was reported.
Acknowledgments
We thank Philip Bastable for his assistance in the revision of the manuscript.
Footnotes
Published online May 14, 2013.
- © 2013 by the Society of Nuclear Medicine and Molecular Imaging, Inc.
REFERENCES
- Received for publication September 26, 2012.
- Accepted for publication January 31, 2013.