Abstract
Antiangiogenic therapy may prolong the dormancy of cancer lesions. Moreover, radioimmunotherapy (RIT) may eradicate this population of cells. This study dealt with determining the benefits associated with the combined usefulness of these 2 therapies with respect to inhibition of tumor growth. Methods: Antiangiogenic therapy using oral thalidomide (daily dose, 200 mg/kg) and RIT involving a single intravenous injection (4.63 MBq 131I-A7, an IgG1 murine monoclonal antibody) were conducted in mice bearing LS180 human colon cancer xenografts. RIT with an irrelevant IgG1, HPMS-1, was also performed as a control. Antiangiogenesis of thalidomide was investigated by immunohistochemical analysis of tumor sections. Results: Antiangiogenic therapy and RIT with 131I-A7 significantly suppressed the growth of xenografts. This combination produced more efficient tumor growth inhibition than did the monotherapy (P < 0.005). RIT using 131I-HPMS-1 was far less effective than 131I-A7, even when combined with thalidomide administration. Immunohistochemistry revealed a decrease in the microvessel number within tumors treated with thalidomide (P < 0.0001). Combined therapy further reduced the microvessel number (P < 0.01 vs. thalidomide monotherapy). Conclusion: The combination of RIT and thalidomide antiangiogenic therapy produces a better response of tumors than does monotherapy. Acting in concert, antiangiogenic therapy may prolong the dormancy of cancer lesions and RIT may eradicate this population of cells.
Theoretically, radioimmunotherapy (RIT) may selectively treat cancer lesions; moreover, systemic administration of radiolabeled monoclonal antibody (mAb) may enable the treatment of disseminated metastatic lesions. However, results reported for conventional RIT in most solid tumors, with the exception of malignant lymphoma, have not been favorable (1). Consequently, modification of RIT is considered to effect killing of solid tumors (1).
Tumor growth depends on neoangiogenesis; therefore, antiangiogenic therapy using agents that suppress endothelial cell growth has been investigated extensively (2). The objective of this treatment is to maintain lesions at limited size, a situation referred to as tumor dormancy (2). We hypothesize that the combined usefulness of antiangiogenic therapy and RIT may synergistically effect treatment of cancer lesions. The rationale for this hypothesis is based on the suppression of tumor endothelial compartments by antiangiogenic agents as well as the direct action of RIT against tumor cells. In addition, antiangiogenic therapy induces dormancy of lesions and RIT may eradicate this population of dormant cells.
Thalidomide was developed as a sedative; however, it was subsequently withdrawn from clinical use because of its teratogenicity. This agent is now known to exert effects on the immune system. Thalidomide inhibits the production of tumor necrosis factor-α, stimulates cytotoxic T-cell proliferation, and induces secretion of interferon-γ and interleukin-2 (3,4). On the basis of its antiinflammatory properties, thalidomide is used in the treatment of such diseases as erythema nodosum leprosum and graft-versus-host disease. In addition, thalidomide possesses potent antiangiogenic properties (5–7), which arise as a result of blockade of the action of angiogenic factors such as vascular endothelial growth factor (VEGF) (8) and basic fibroblast growth factor (bFGF) (5). Consequently, this agent is being investigated currently in clinical trials of patients exhibiting various types of malignancies (3,4). This study dealt with determining the effects of combined RIT and thalidomide therapy in the treatment of xenografts of human colon cancer cells.
MATERIALS AND METHODS
mAb and Tumor Model
A7, an IgG1 murine mAb with κ-light chain, was used in this investigation (9). It was purified from ascites of hybridoma-bearing mice by protein A-Sepharose column (Bio-Rad, Richmond, CA) chromatography. A7 mAb recognizes a 45-kDa surface glycoprotein of human colonic carcinomas. An additional IgG1 recognizing placental alkaline phosphatase, HPMS-1, was used as a class-matched irrelevant control mAb (10). The mAbs were radiolabeled with 131I by the chloramine-T method and subsequently purified on a PD-10 column (Pharmacia LKB Biotechnology, Uppsala, Sweden). The specific activity of purified 131I-A7 was 142 MBq/mg. Immunoreactivity of purified 131I-A7 was assessed as 69% in antigen excess conditions with an LS180 human colon cancer cell line. The specific activity of 131I-HPMS-1 was 90 MBq/mg. Antibodies were sterilized by filtration (Millex-GV, 0.22 mm; Millipore, Bedford, MA) before further experiments. Animal studies were performed in compliance with the regulations of our institutions, and the principles of laboratory animal care were followed. BALB/c nu/nu mice (female, 6–8 wk old) were xenografted subcutaneously in the thigh area with 5 × 106 LS180 cells. Animals were subjected to treatment when xenografts attained a diameter of approximately 5–7 mm. This cell line reportedly shows considerable VEGF production; moreover, the growth of xenografts in mice is suppressed by an anti-VEGF antibody (11).
Antitumor Effect of 131I-A7 with Thalidomide
Tumor volume (mm3), calculated as length (mm) × width (mm)2 × 0.5, was expressed as a ratio of volumes to the volume obtained on the initial day of treatment (day 0). Tumor volume on day 0 was measured as 139 ± 21 mm3. The therapeutic experiment consisted of 6 groups: nontreated mice (n = 5); mice receiving a daily oral dose of 200 mg/kg thalidomide (n = 10); mice undergoing treatment with 4.63 MBq 131I-A7 (n = 10); mice treated with 131I-A7 and a daily dose of thalidomide (n = 10); mice treated with 4.63 MBq of an irrelevant mAb, 131I-HPMS-1 (n = 5); and mice receiving 131I-HPMS-1 and a daily dose of thalidomide (n = 6). Thalidomide suspensions were prepared in 0.1 mL 0.5% carboxymethylcellulose (Wako, Osaka, Japan) and administered orally. The dose of 131I-A7 used in this study was approximately half of the maximum tolerated dose in this model. Thalidomide dosage was determined in accordance with previous reports (5–7). This dosage has not been reported to cause critical side effects. Nontreated mice and mice receiving RIT monotherapy were orally administered an identical volume of carboxymethylcellulose that did not contain thalidomide. The therapeutic efficacy of each treatment was analyzed statistically by tumor size on day 13. Furthermore, analysis with the area under the curve (AUC) of tumor growth curves, which would be the reflection of the therapeutic effect during the entire observation period, was also performed for the comparison among the treatment groups.
Toxicity of Treatment
A major critical organ in RIT is the bone marrow. Thalidomide may cause neutropenia (3). Therefore, we attempted to establish whether the combined therapy increased myelotoxicity in animals. Nontumor-bearing mice were partitioned into 4 groups: nontreated mice, mice receiving RIT (4.63 MBq), mice treated with a daily dose of thalidomide (200 mg/kg), and mice undergoing the combined regimen (n = 3). Thalidomide administration was conducted until day 23. A blood sample (5 μL) was obtained from a tail vein in each mouse. Samples from mice within a single group were pooled and diluted 1:10,000 in phosphate-buffered saline for red blood cell (RBC) counts, 1:20 in 3% acetic acid for white blood cell (WBC) counts, and 1:100 in 1% ammonium oxalate for platelet counts (12). Body weight of the animals was also determined.
Assessment of Microvessel Density in Tumors
Tumors were excised from nontreated control mice, mice treated with either thalidomide or 131I-A7 RIT monotherapy, and mice treated with the combined therapy on day 15. Specimens were fixed with 10% formalin and embedded in paraffin wax (n = 3). Immunoperoxidase staining using a rabbit polyclonal antibody against factor VIII (DAKO, Copenhagen, Denmark) was performed. The microvessel density was determined by light microscopy according to the procedure described by Weidner et al. (13). Briefly, the areas of highest vascularization were detected by low-power scanning (100×) of the tumor sections. Individual microvessels were then counted in a 200× field, with 5 different fields examined in each section. Two sections per tumor were studied, and the average of 3 tumors in each group was compared.
In Vitro Assay with Thalidomide
Cells were incubated in 24-well culture plates at 20,000 cells per milliliter in Dulbecco’s modified Eagle medium (Nissui Seiyaku, Tokyo, Japan) to determine whether thalidomide would exert a direct effect on LS180 cell growth. Increasing concentrations of thalidomide (up to 100 μmol/L) were introduced. The agents were dissolved in dimethyl sulfoxide ([DMSO]; Wako, Tokyo, Japan) and diluted in the medium. The final concentration of DMSO was 0.5%. Control cells were treated with DMSO alone. Before the assay, confirmation was obtained that DMSO at this concentration did not affect proliferation of LS180 cells. Cells were exposed to trypsin, and the viable cell number was then determined 1, 3, and 5 d after inoculation (n = 3).
The possibility of radiosensitization effects of thalidomide on LS180 cells was investigated. Briefly, cells were treated with thalidomide at various concentrations up to 100 μmol/L for 24 h, followed by exposure to an x-ray beam (0–8 Gy) (MBR-1520R; Hitachi, Tokyo, Japan) at 1.41 Gy/min. Cell proliferation was observed, and the surviving fraction was calculated as described (14).
Statistical Analysis
Results were analyzed by 1-way ANOVA with Fisher’s protected least-significant difference. The level of significance was set at 5% .
RESULTS
Either thalidomide or 131I-A7 RIT monotherapy significantly suppressed growth of LS180 xenografts in comparison with no treatment (P < 0.005 and P < 0.0005, respectively) (Fig. 1). Relative tumor volumes on day 13 were 12.7 ± 2.95, 6.84 ± 0.71, and 3.05 ± 0.37 in the control group, the group administered thalidomide, and the group receiving 131I-A7 RIT, respectively. Treatment with a class-matched irrelevant mAb, 131I-HPMS-1, did not effectively suppress tumor growth (P = 0.06). The combined treatment of thalidomide and 131I-A7 RIT resulted in more efficient tumor growth inhibition than did monotherapy with thalidomide or RIT alone, displaying a tumor volume of 1.31 ± 0.33 on day 13 (P < 0.005 vs. RIT alone). Moreover, 6 of 10 tumors regressed with combined treatment, whereas no regression occurred with monotherapy. Results of AUC analysis of tumor growth curves also indicated the superiority of the combined regimen of thalidomide and 131I-A7 RIT over monotherapy for the inhibition of tumor growth (Table 1). The combination of 131I-HPMS-1 and thalidomide was far less effective than the combination of 131I-A7 and thalidomide.
Growth of LS180 colon cancer xenografts. Tumor volume is expressed as ratio relative to volume on day 0. ♦, Nontreated control; ▴, tumors treated with 200 mg/kg/d thalidomide; □, tumors treated with 4.63 MBq 131I-HPMS-1; ▪, tumors treated with thalidomide and 131I-HPMS-1; ○, tumors treated with 4.63 MBq 131I-A7; •, tumors treated with thalidomide and 131I-A7.
Analysis of AUC of Growth Curves of Colon Cancer Xenografts After Initiation of Treatment
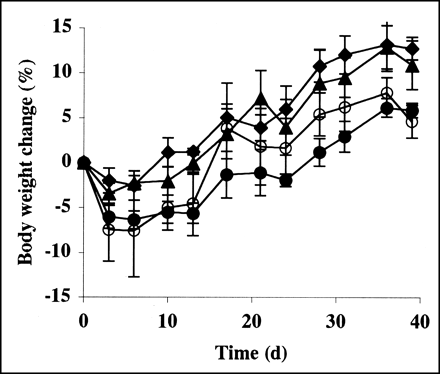
Thalidomide treatment induced minor depression of the peripheral WBC count relative to that observed in nontreated mice (Fig. 2). The nadir of WBC count depression in mice treated with the combination of RIT and thalidomide therapy was similar to that in mice treated with RIT alone; however, the depression persisted in mice treated with the combination regimen. The WBC count recovered after the cessation of thalidomide administration. Changes in the platelet count in mice receiving the combination treatment did not vary from those in mice undergoing RIT alone. Thalidomide administration did not affect the RBC count. The use of thalidomide did not result in weight loss that was different than that of the control (Fig. 3). The maximal body weight loss was similar in mice treated with RIT alone and those undergoing the combination regimen; however, recovery was delayed by thalidomide treatment. No mice showed signs of diarrhea or infection.
Changes in peripheral blood cell counts in mice. (A) RBCs; (B) WBCs; (C) platelets. ♦, Nontreated control; ▴, tumors treated with 200 mg/kg/d thalidomide; ○, tumors treated with 4.63-MBq RIT; •, tumors treated with thalidomide and RIT.
Body weight changes of mice. ♦, Nontreated control; ▴, tumors treated with 200 mg/kg/d thalidomide; ○, tumors treated with 4.63-MBq RIT; •, tumors treated with thalidomide and RIT.
Immunostaining of tumor sections with antifactor VIII antibody showed significant reduction in the microvessel density in tumors treated with thalidomide (8.30 ± 0.8 per 200× field) in comparison with nontreated control tumors (29.9 ± 2.5) (P < 0.0001) (Fig. 4). Interestingly, the microvessel density in tumors treated with 131I-A7 RIT (10.8 ± 1.4) was lower than that in control tumors (P < 0.0001). Moreover, the combination of RIT and thalidomide treatment further decreased the microvessel density in tumors (4.3 ± 0.5; P < 0.0001 vs. RIT monotherapy and P < 0.01 vs. thalidomide monotherapy).
Immunohistochemistry of tumor sections with an antifactor VIII antibody. Microvessel numbers in 200× field are 29.9 ± 2.5 in control tumor (A) and 8.30 ± 0.8 in tumor treated with thalidomide (B) (P < 0.0001).
The in vitro proliferation assay revealed no evidence of direct cytotoxicity of thalidomide in LS180 cells up to concentrations of 100 μmol/L (Fig. 5). In addition, thalidomide did not radiosensitize the cells (Fig. 6).
In vitro proliferation assay of LS180 colon cancer cells in presence of increasing concentrations of thalidomide (n = 3). ▪, Without thalidomide; in presence of thalidomide: •, 0.1 μmol/L; ▴, 1 μmol/L; ♦, 5 μmol/L; □, 10 μ mol/L; ○, 50 μmol/L; ▵, 100 μmol/L.
Clonogenic assay of LS180 colon cancer cells exposed to x-ray radiation (n = 3). ♦, Control cells; cells pretreated with thalidomide for 24 h: ▪, 1 μmol/L; ▴, 10 μmol/L; ✖, 100 μmol/L.
DISCUSSION
Thalidomide is currently being investigated in several clinical trials involving tumors such as low-grade glioma, AIDS-related Kaposi’s sarcoma, renal cell carcinoma, hepatocellular carcinoma, and multiple myeloma (3). The effectiveness of thalidomide in colorectal cancer patients is also currently being evaluated (3). It is known that the degree of tumor vascularization is a prognostic factor of colon cancer and is correlated with its metastases and recurrence (15). Expression of VEGF and bFGF also correlates with prognosis in colon cancer patients (16). A neutralizing anti-VEGF mAb exhibits antitumor activity in xenografts of various colon cancer cell lines (11). In our study, the tumor-suppressive effects of thalidomide were detected in xenografts of LS180 colon cancer cells. These facts suggest a role for antiangiogenic therapy in patients with colon cancer. However, because the response rates of solid tumors to monotherapy with thalidomide are limited in clinical trials, the combination with chemotherapy or radiotherapy has been considered. The efficacy of conventional RIT is also currently limited in most types of tumors; consequently, several methods have been proposed to improve its therapeutic outcome (1).
The combination of antiangiogenic therapy and RIT proposed in this investigation is a novel approach in which 2 agents act against distinct compartments within tumors: Antiangiogenic agents suppress the endothelial compartment, whereas RIT acts directly against the tumor cell. We showed that thalidomide was unable to exert direct cytotoxicity on LS180 cells or to sensitize cells to radiation; however, thalidomide reduced the microvessel density in xenografts in mice. These results clearly indicate that the cooperative improvement of tumor growth suppression with combined therapy was related to this hypothesis. The effectiveness of combining RIT and another antiangiogenic agent, 2-methoxyestradiol, has been reported recently (17).
Interestingly, 131I-A7 RIT monotherapy reduced the microvessel number in tumors compared with no treatment; furthermore, the combined regimen more effectively decreased the microvessel number than did thalidomide monotherapy. These findings were probably caused by radiation injury of the vessels due to 131I-A7. Because penetration of extravasated mAbs into solid tumors is disturbed by factors such as high interstitial pressure (18) and the so-called binding site barrier (19), mAbs would deposit close to the vasculature; for instance, IgG would require days to cover the distance of ∼1 mm after the extravasation (18). Consequently, considerable radiation dose would be absorbed by the vascular compartment. In this sense, RIT and antiangiogenic therapy would cooperatively give effect to suppression of the tumor vasculature in the combined regimen.
Changes in peripheral blood cell counts and the body weight of mice were monitored to assess the toxicity of treatment. In addition to teratogenicity, thalidomide displays several adverse effects, including somnolence, constipation, skin rashes, orthostatic hypotension, nausea, peripheral neuropathy, and neutropenia (3, 4). Most of these untoward effects do not occur in RIT therapy; however, neutropenia may affect combination with RIT. Minor depression of the peripheral WBC count was observed in mice that were treated daily with thalidomide. The combined regimen delayed recovery of the WBC count in comparison with RIT monotherapy. Recovery of body weight loss was also delayed by this combination. Reports describing clinical trials of thalidomide indicate that thalidomide-induced neutropenia occurs rarely in patients other than those presenting with HIV infection and recipients of an allogeneic bone marrow transplant (3,4); however, marrow toxicity should be carefully monitored during combination therapy involving RIT and thalidomide.
Clinical results of RIT have been disappointing in the treatment of bulky disease of solid tumors (1). However, several reports have described the role of RIT in an adjuvant setting of cancer treatment, in which small residual tumors are targeted by mAbs (20–23). Antiangiogenic therapy also has an important role in this setting, in which angiogenesis inhibitors may induce dormancy of microscopic metastases or stabilization of residual disease (24–26). Because antiangiogenic therapy alone may not produce a complete cure, a combination with cytotoxic therapy is required to be curative (1). In this regard, a combined regimen with external beam radiotherapy has been proven to enhance tumor growth suppression in various kinds of tumors (27,28). However, as a local treatment, external beam radiotherapy should have a limitation in patients with metastatic foci. The combined regimen with RIT and antiangiogenic therapy surely has an advantage in this setting, because both treatments can be administered systemically.
CONCLUSION
We observed a cooperative relationship in the combined usefulness of RIT and antiangiogenic therapy with thalidomide in the treatment of colon cancer xenografts. Acting in concert, antiangiogenic therapy may prolong the dormancy of cancer lesions and RIT may eradicate this population of cells. We are currently testing this hypothesis in liver metastasis and intraperitoneal metastasis models.
Acknowledgments
The authors thank former Professor Toshio Takahashi and Dr. Toshiharu Yamaguchi (First Department of Surgery, Kyoto Prefectural University of Medicine) for providing A7 mAb. This study was supported in part by grants-in-aid from the Ministry of Education, Science, Sports and Culture, Japan.
Footnotes
Received Oct. 29, 2001; revision accepted Apr. 19, 2002.
For correspondence or reprints contact: Seigo Kinuya, MD, Department of Biotracer Medicine, Kanazawa University Graduate School of Medical Sciences, 13-1 Takaramachi, Kanazawa, Ishikawa 920-8640, Japan.
E-mail: kinuya{at}med.kanazawa-u.ac.jp