Abstract
Several radiolabeled chemotactic peptides have been tested for their suitability to show infection and inflammation. Leukotriene B4 (LTB4) receptor-binding ligands could be useful agents for revealing neutrophilic infiltrations because the LTB4 receptor is abundantly expressed on neutrophils after an inflammatory stimulus. In this study, we investigated the in vivo and in vitro characteristics of a new hydrophilic 111In-labeled LTB4 antagonist. Methods: The LTB4 antagonist DPC11870-11 was labeled with 111In and intravenously injected into New Zealand White rabbits with Escherichia coli infection in the left thigh muscle. The pharmacokinetics and biodistribution were studied by serial scintigraphic imaging (0–24 h after injection) and by ex vivo counting of dissected tissues (6 and 24 h after injection). The receptor-mediated in vivo localization of the compound was investigated in 3 rabbits that received an excess of nonradioactive indium-labeled agent 2 min before the administration of the 111In-labeled LTB4 antagonist. Results: In rabbits with intramuscular E. coli infection, the abscess was visualized as early as 2 h after injection. Accumulation in the abscess increased with time, resulting in excellent images at 6 h after injection. Blood clearance was rapid in the first hours after injection (α-half-life = 30 ± 6 min, 85%; β-half-life = 25.7 ± 0.8 h, 15%). Abscess-to-background ratios, as derived from the region-of-interest analysis, increased to 34 ± 7 at 24 h after injection. The images of both groups showed moderate uptake in the liver, spleen, kidneys, and bone marrow. No activity was seen in the bladder, indicating almost complete retention in the kidneys. The uptake in the abscess could be blocked completely by injection of an excess of nonradioactive agent, indicating a specific receptor-ligand interaction of the radiolabeled agent in the infected tissue. Biodistribution data showed that after saturation of the LTB4 receptor, the abscess uptake, in percentage injected dose per gram, was significantly reduced (0.03 ± 0.02 vs. 0.24 ± 0.06, P = 0.008). Conclusion: The modified LTB4 antagonist showed infectious foci rapidly after injection because of specific receptor-ligand interaction. Because of the high abscess-to-background ratios that were obtained and the fact that no accumulation of radioactivity was observed in the gastrointestinal tract, this compound has excellent characteristics for revealing infectious and inflammatory foci.
For the scintigraphic imaging of infection and inflammation, the use of radiolabeled autologous leukocytes is considered the gold standard. Because preparation of radiolabeled white blood cells is laborious and requires handling of potentially contaminated blood, there is an ongoing search for an agent that can show infectious and inflammatory foci and be prepared instantaneously (1). Ideally, such a radiopharmaceutical preferably would be synthesized chemically instead of using material with a biologic origin. Furthermore, these compounds would be easily and rapidly radiolabeled and would not provoke immunologic responses (2). Several compounds, such as radiolabeled antibodies, liposomes, and receptor-binding peptides, have been tested for this application (3,4).
The use of radiolabeled chemotactic peptides as an infection-imaging agent is based on the enhanced expression of high-affinity receptors on infiltrating granulocytes activated after an inflammatory response. Several of these peptides (chemokines, platelet factor 4, N-formyl-methionyl-leucyl-phenylalanine, and other chemotactic compounds) have been studied for the detection of infection or inflammation (5).
In this study, we examined the characteristics of an 111In-labeled leukotriene B4 (LTB4) antagonist for its potential to reveal infectious foci scintigraphically. LTB4 is a potent chemoattractant that activates granulocytes and macrophages as a reaction to an inflammatory response (6,7). At the site of the inflammation, enhanced LTB4 synthesis occurs mainly by leukocytes from arachidonic acid through the 5-lipoxygenase pathway (8). LTB4 responses are receptor mediated, and 2 classes of stereospecific binding sites for LTB4 have been identified. The first type of receptor, BLT1, is a high-affinity receptor (dissociation constant for LTB4 is 1 nmol/L) mainly expressed on human neutrophils (9). The second receptor type, BLT2, is a low-affinity receptor (dissociation constant for LTB4 is 23 nmol/L) that is more ubiquitously expressed (6,10). Binding of LTB4 to BLT1 and BLT2 receptors promotes chemotaxis and chemokinesis. Therefore, LTB4 is considered an important mediator in both acute and chronic inflammatory diseases (9,11).
In a previous study, we showed the potential of a 99mTc-labeled LTB4 antagonist, RP517, to visualize experimental infection (12–14). The disadvantage of 99mTc-RP517, however, was its hepatobiliary clearance, causing high uptake in the organs of the digestive tract relatively early after injection. This gastrointestinal uptake of 99mTc-RP517 limits its applicability as an infection-imaging agent (12).
Because the radiolabeled LTB4 antagonist showed rapid and high accumulation in the infected tissue, we produced a hydrophilic bivalent LTB4 antagonist conjugated with a diethylenetriaminepentaacetic acid (DTPA) moiety to allow radiolabeling with 111In. In the present study, the leukocyte-binding characteristics of this modified bivalent LTB4 antagonist were studied both in vitro and in vivo.
MATERIALS AND METHODS
DPC11870-11 Synthesis
DPC11870-11 (Fig. 1) is a bivalent LTB4 antagonist consisting of 2 identical LTB4 receptor-binding moieties each modified with tetra-cysteic acid as pharmacokinetic modifiers. The LTB4-binding moieties are joined through the carboxyl groups of glutamic acid and conjugated with DTPA.
Structural formula of DPC11870-11.
A complete description of the synthesis of DPC11870-11 will be published elsewhere. Briefly, the tetra-cysteic acid derivative of SG385, prepared as reported previously (15), was dimerized by reaction with the bis-tetrafluorophenol ester of Boc-glutamic acid. Removal of the Boc group and conjugation with DTPA anhydride gave crude DPC11870-11. The product was further purified by reverse-phase high-performance liquid chromatography (RP-HPLC). RP-HPLC analysis on a C18 column eluted with a 0.5%/min acetonitrile gradient of the purified and lyophilized product gave a single peak. The purified DPC11870-11 was characterized further by nuclear magnetic resonance and mass spectrometry.
Radiolabeling of DPC11870-11
Labeling of DPC11870-11 with 111In was performed in metal-free 0.25 mol/L ammonium acetate buffer, pH 5.5, for 30 min at room temperature. Labeling experiments were performed to determine the maximum specific activity of the radiolabeled agent. Radiochemical purity was checked by instant thin-layer chromatography on silica gel strips (Gelman Sciences, Inc.) in 0.1 mol/L sodium citrate buffer, pH 6.0. Strips were analyzed in a well-type γ-counter (Wizard; Pharmacia-LKB). In addition, RP-HPLC was performed on a C18 column (Rx-C18, 4.6 mm × 25 cm; Zorbax) on an Agilent 1100 system equipped with an in-line radiodetector (Canberra Packard). During analysis, a gradient was used from 100% solvent NH4Ac, pH 7.0, to 100% acetonitrile in 50 min, at a flow rate of 1 mL/min.
Labeling with nonradioactive InCl3 (Aldrich) was performed under the same conditions as described above. A 3-fold molar excess of InCl3 was added to DPC11870-11 in ammonium acetate buffer, pH 5.5.
Receptor-Binding Assay
In vitro binding studies were performed on purified human granulocytes. The granulocytes were purified as described previously (16). To 1 × 108 cells, increasing amounts of nonradioactive indium-DPC11870-11 (0–10 μmol/L) were added in the presence of 10,000 cpm 111In-DPC11870-11 (0.4 nmol/L) in 0.25 mol/L Tris/HCl, pH 7.2. After incubation during 1 h at 37°C, cells were washed twice (5 min, 5,000g), the supernatant was discarded, and the radioactivity in the pellet (total bound activity) was measured in a shielded well-type γ-counter (Wizard).
The specifically bound fraction versus the nonradioactive LTB4 antagonist concentration was plotted. The 50% inhibitory concentration (IC50) was determined as being the concentration of nonradioactive indium-DPC11870-11 that caused 50% inhibition of the maximum binding of the 111In-DPC11870-11.
Infection Model
Thirteen female New Zealand White rabbits weighing 2.3–2.8 kg were kept in cages (1 rabbit per cage) and fed standard laboratory chow and water ad libitum. An Escherichia coli infection was induced in the left thigh muscle by intramuscular injection of 4 × 109 colony-forming units of E. coli. During this procedure, the rabbits were anesthetized by subcutaneous injection of 0.7 mL of a mixture of 0.315 mg/mL fentanyl and 10 mg/mL fluanisone (Hypnorm; Janssen Pharmaceutica). All animal experiments were approved by the local animal welfare committee in accordance with the Dutch legislation and performed in accordance with their guidelines.
Imaging and Biodistribution
Twenty-four hours after induction of the infection, when swelling of the infected muscle was apparent, 13 rabbits were intravenously injected with 11 MBq of 111In-DPC11870-11 (3 μg) in the lateral ear vein. Three rabbits received 2 mg of nonradioactive indium-DPC11870-11 2 min before the injection of the radiolabeled compound. The excess of nonradioactive agent was injected to induce saturation of the LTB4 receptors in vivo. This experiment was performed to determine receptor-specific accumulation of 111In-DPC11870-11 in the abscess.
For scintigraphic imaging, the rabbits were immobilized in a mold and placed prone on a gamma camera (Orbiter; Siemens) using a medium-energy parallel-hole collimator. Images (300,000 counts per image) were obtained up to 24 h after injection and stored digitally in a 256 × 256 matrix. All images were windowed identically, allowing a fair comparison among the various experiments. The scintigraphic results were analyzed by drawing regions of interest over the abscess and the contralateral muscle (background). Abscess-to-contralateral muscle ratios were calculated.
The 3 rabbits that received an excess of nonradioactive agent and the 5 rabbits injected only with radiolabeled antagonist were euthanized at 6 h after injection with a lethal dose of sodium phenobarbital to determine the biodistribution of the agent. At 24 h after injection, the other 5 rabbits were euthanized. A blood sample was taken by cardiac puncture. Tissues were dissected and weighed. The activity in tissues was measured in a shielded well-type γ-counter together with the injection standards and was expressed as the percentage injected dose (%ID) per gram.
Pharmacokinetics
The pharmacokinetics of the 111In-labeled agent were studied in detail in 2 rabbits that were injected with the 111In-DPC11870-11 and 2 rabbits that received an excess of nonradioactive DPC11870-11 before the 111In-DPC11870-11. Blood samples were drawn at 1 min before and 3, 15, 30, 90, 120, 240, 360, and 1,440 min after injection. The activity in the samples was determined in a γ-counter and expressed as %ID assuming that the total blood weight accounted for 6% of total body weight (17). The α- and β-half-lives (t½α and t½β, respectively) were calculated assuming a 2-phase linear model for the blood clearance. In addition, blood samples were divided into 2 portions; the first tube was used for white blood cell analysis; the second tube was centrifuged (5 min, 1,500g), and activity in the pellet and plasma was determined. 111In-DPC11870-11 and the plasma samples were analyzed by fast protein liquid chromatography (FPLC) on a Biosep 3000 gel filtration column (Phenomenex), using phosphate-buffered saline, pH 7.2, as eluent, at a flow rate of 1 mL/min.
Statistical Analysis
All mean values are presented as mean ± SD. Statistical analysis was performed using the 2-sided Student t test. The level of significance was set at 0.05.
RESULTS
Radiolabeling and Receptor-Binding Studies
The RP-HPLC radioactivity elution profile showed that the unbound 111In eluted with the void volume with a retention time of 2.5 min, whereas the labeled compound eluted with a retention time of 30.1 min (51% acetonitrile) (Fig. 2).
HPLC radiogram of 111In-DPC11870-11 using C18 RP-HPLC column eluted with 5%/min acetonitrile gradient.
RP-HPLC analysis showed that the maximum specific activity achieved with a labeling efficiency exceeding 95% was 3.7 MBq/μg DPC11870-11 (12 MBq/nmol).
The IC50 of DPC11870-11 was 10 nmol/L. Nonspecific binding was determined as the binding of 111In-DPC11870-11 to granulocytes in the presence of 10 μmol/L nonradioactive indium-DPC11870-11. The nonspecific binding of 111In-DPC11870-11 to the granulocytes was relatively high (40%). The competitive binding assay was also performed using human lymphocytes and erythrocytes. 111In-DPC11870-11 did not bind specifically to these cell types. The amount of 111In-DPC11870-11 bound to these cells (incubated in the presence and absence of nonradioactive indium-DPC11870-11) did not exceed 5%.
Imaging and Biodistribution
The scintigraphic images after the injection of 111In-DPC11870-11 are shown in Figure 3. Immediately after injection of the radiolabeled LTB4 antagonist, an accumulation of radioactivity was observed in the lung, liver, and kidneys. Subsequently, an accumulation of activity was seen in the spleen and bone marrow.
(A) Scintigraphic images of E. coli infection in thigh muscle of a rabbit. The rabbit received 11 MBq of 111In-DPC11870-11 (3 μg) intravenously. Anterior images (from left to right) were acquired immediately and at 2, 6, and 24 h after injection of 111In-DPC11870-11. (B) Scintigraphic image of rabbit that received 2 mg of nonradioactive indium-DPC11870-11 before injection of 111In-labeled LTB4 antagonist. This anterior image was acquired 6 h after injection of radiolabel.
As early as 2 h after injection, the infectious foci were visualized. Visualization of the abscess improved with time. At 24 h after injection, the abscess and kidneys were the tissues with the highest activity concentration. No activity was seen in the bladder at any time. In addition, the digestive tract was not visualized, indicating that the compound was not cleared through the hepatobiliary route (Fig. 3A). Abscess-to-contralateral muscle ratios as derived from region-of-interest analysis of the images increased significantly between 6 h after injection (18 ± 4) and 24 h after injection (34 ± 7) (P = 0.022). In the rabbits preinjected with an excess of nonradioactive indium-DPC11870-11, the infected thigh muscle was not visualized, indicating that abscess uptake of 111In-DPC11870-11 was receptor mediated (Fig. 3B).
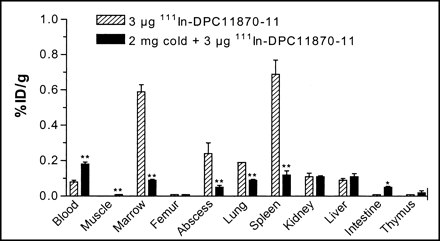
The biodistribution data derived from ex vivo counting of dissected tissues, as summarized in Figure 4, were consistent with the scintigraphic images. Uptake of 111In-DPC11870-11 in the abscess at 6 h after injection was 0.24 ± 0.06 %ID/g and remained constant until 24 h after injection (0.25 ± 0.02 %ID/g). The radioactivity concentrations in the blood at 6 and 24 h after injection were 0.09 ± 0.03 %ID/g and 0.03 ± 0.01 %ID/g, respectively. Uptake in the bone marrow and spleen was relatively high (0.59 ± 0.04 %ID/g and 0.69 ± 0.08 %ID/g, respectively, at 6 h after injection), whereas uptake in the other tissues was relatively low.
Biodistribution data obtained 6 h after injection of 111In-DPC11870-11. Each bar represents mean value ± SD. Values were analyzed using unpaired t test. *P < 0.05 and **P < 0.01 for differences in uptake of radiolabel between rabbits injected also with 2 mg of nonradioactive LTB4 antagonist and rabbits injected only with radiolabeled compound (3 μg).
In the rabbits preinjected with an excess of nonradioactive indium-DPC11870-11, the uptake of radiolabel in the abscess was significantly lower (0.03 ± 0.02 %ID/g vs. 0.24 ± 0.06 %ID/g, P < 0.004). Uptake in the spleen and bone marrow was also significantly lower in these animals (0.012 ± 0.04 %ID/g vs. 0.69 ± 0.08 %ID/g, P < 0.0003, and 0.09 ± 0.01 %ID/g vs. 0.59 ± 0.04 %ID/g, P < 0.002, respectively). The blood levels in these animals were significantly higher (0.18 ± 0.02 %ID/g vs. 0.09 ± 0.03 %ID/g, P < 0.004).
Pharmacokinetics
Figure 5 depicts the radioactivity concentrations in the blood after injection of 111In-DPC11870-11. In the first hours after injection of the radiolabeled compound, the clearance was fast (t½α = 30 ± 6 min, 85%); later, the compound cleared from the blood much more slowly (t½β = 25.7 ± 0.8 h, 15%). Considering the molecular weight of the agent, the t½β is remarkably high, suggesting an association with blood cells or serum proteins. Measurement of plasma samples demonstrated that more than 98% of the radioactivity was found in the plasma (Fig. 5). FPLC analysis of plasma samples on a gel filtration column showed 1 radioactive peak at 11 min, coeluting with proteins with a molecular weight of 50–100 kDa. Incubation of the 111In-labeled compound in phosphate-buffered saline with 0.5% bovine serum albumin and analysis with FPLC on a gel filtration column resulted in elution patterns with 1 activity peak with a retention time corresponding to bovine serum albumin (11 min). Neither the rabbits injected with 111In-DPC11870-11 nor the animals injected with 111In-DPC11870-11 and an excess of nonradioactive compound showed significant changes in white blood cell counts. As shown in Figure 6, the white blood cell concentration before injection of the 111In-DPC11870-11 was 7.6 ± 3.3 × 109/L, and at all time points after injection the cell concentration was within the normal range (3.2–13.2 × 109/L). This finding indicated that this LTB4 antagonist did not induce changes in peripheral leukocyte counts.
Blood clearance of 111In-DPC11870-11 in New Zealand White rabbits. Average amount and SD of activity for 2 rabbits are expressed as %ID/g present in blood and plasma. Plasma concentration was corrected using hematocrit of animals.
White blood cell counts after injection of 111In-DPC11870-11. Initial amount of white blood cells is represented as 100%. Number of cells determined after injection is calculated as percentage of this initial value.
DISCUSSION
The present studies demonstrated that DPC11870-11 can be labeled with 111In easily and efficiently, resulting in a high specific activity and high radiochemical purity. The IC50, determined at 10 nmol/L, indicated that 111In-DPC11870-11 binds to its receptor with high affinity. The in vitro receptor-binding results suggest that 111In-DPC11870-11 accumulates in infected tissue by binding to LTB4 receptor-positive cells. The in vivo imaging experiments in rabbits showed that the 111In-labeled LTB4 antagonist could already reveal E. coli infection a few hours after the injection. Because of rapid blood clearance in the first hours after the injection, low background activity was seen that, in combination with the rapid accumulation at the site of infection, resulted in excellent visualization of the infectious foci at 6 h after injection. The activity concentration in the infectious foci did not increase from 6 to 24 h. Improvement of contrast in the images at 24 h after injection was due to the ongoing activity clearance from the background. The relatively high uptake of radiolabel in the bone marrow is remarkable. Apparently, the compound easily enters the bone marrow compartment, where it may bind to LTB4 receptor-positive cells.
Administration of an excess of nonradioactive indium-labeled compound indicated that the targeting of infectious foci was a result of specific receptor-ligand interaction. The in vivo receptor-blocking experiments also indicated that the targeting of cells in the bone marrow and spleen was dependent on interaction with receptor-positive cells. These findings are in accordance with those of Yokomizo et al. (18), who showed that highest expression of human BLT1 messenger RNA was observed in leukocytes, followed by cells in the spleen and thymus.
Unlike the images obtained with the more lipophilic agent 99mTc-RP517 as reported previously, the images obtained with 111In-DPC11870-11 showed no accumulation in the gastrointestinal tract (12). Abscess uptake for 111In-DPC11870-11 was higher at all times, compared with that for 99mTc-RP517 (0.035 ± 0.007 %ID/g at 4 h after injection; 0.12 ± 0.02 %ID/g at 20 h after injection). In contrast, blood levels of 99mTc-RP517 were lower (0.024 ± 0.004 %ID/g at 4 h after injection; 0.004 ± 0.0004 %ID/g at 20 h after injection), resulting in lower background activity. In this animal model, abscess-to-contralateral muscle ratios were in the same range for both agents.
Pharmacokinetic analysis showed that the compound cleared from the blood rapidly in the first hours after administration. The distribution half-life (t½α) was in accordance with the low molecular weight of the compound. A fraction of the radiolabeled agent (15%), however, seemed to clear more slowly from the blood (t½β = 25.7 h), which is unusual for a low-molecular-weight compound (molecular weight, 3,127 Da). This slow clearance may be due to interaction of the radiolabeled agent with serum proteins (19). The FPLC analysis of plasma samples indeed showed that 111In-DPC11870-11 interacted with albumin.
Radiolabeled chemotactic peptides have been intensively studied for their applicability to the imaging of infectious and inflammatory foci. Their theoretically high affinity for blood cell receptors, good penetrating ability, and rapid background clearance suggest that they are ideal candidates for this application. A main disadvantage of some chemotactic and chemoattractive compounds is the induction of transient leukopenia after intravenous injection (4). The data in this study show that indium-DPC11870-11 does not provoke any of these changes in rabbits, even at very high doses (2 mg). Therefore, it seems unlikely that the LTB4 antagonist used in this study will provoke side effects after intravenous administration in patients.
CONCLUSION
The present study demonstrates that this radiolabeled bivalent LTB4 antagonist meets the most important requirements of a radiopharmaceutical to visualize infection and inflammation. The agent reveals infectious foci within a few hours after injection. The compound can be labeled easily and efficiently, does not provoke hematologic changes in the peripheral blood, and can be produced relatively easily through chemical synthesis.
Because of these findings, further in vitro and in vivo studies are warranted, preferably with an analog that can be labeled with 99mTc. Replacement of the DTPA moiety with a 99mTc-chelating moiety will be attempted.
Acknowledgments
The authors thank Gerry Grutters and Hennie Eijkholt (Central Animal Laboratory, University of Nijmegen) for technical assistance and Peter Mast and colleagues (Central Hematological Laboratory, University Medical Centre Nijmegen) for the blood sample analysis.
Footnotes
Received Sep. 23, 2002; revision accepted Mar. 5, 2003.
For correspondence or reprints contact: Julliëtte E.M. van Eerd, BSc, Department of Nuclear Medicine, University Medical Center Nijmegen, P.O. Box 9101, 6500 HB Nijmegen, The Netherlands.
E-mail: J.vaneerd{at}nucmed.umcn.nl