Abstract
Several studies have shown that 11C-choline PET/CT may be useful for restaging prostate cancer (PCa) patients with biochemical failure after radical prostatectomy. However, validation of 11C-choline PET/CT findings scarcely relied on histologic findings, and prognostic implications of 11C-choline PET/CT are currently unknown. The aim of this study was to assess whether 11C-choline PET/CT predicts survival in PCa patients. Methods: This retrospective study included 195 PCa patients treated with radical prostatectomy who underwent 11C-choline PET/CT from December 1, 2004, to July 31, 2007, due to biochemical failure (prostate-specific antigen > 0.2 mg/mL) during androgen-deprivation therapy. PCa-specific survival was computed as the interval from radical prostatectomy to PCa-specific death. Results: The median interval after radical prostatectomy was 8.9 y (95% confidence interval [CI], 1.7–18.9 y). The median follow-up after 11C-choline PET/CT was 4.5 y (95% CI, 0.4–8.5 y). 11C-choline PET/CT results were positive in 57% of patients. The median PCa-specific survival was 16.4 y (95% CI, 14.0–18.8 y) in patients with negative 11C-choline PET/CT results and 11.2 y (95% CI, 9.8–12.6 y) in patients with positive 11C-choline PET/CT results (log-rank: χ2 = 19.3, P < 0001). At multivariate analysis, statistical significance was obtained for 11C-choline PET/CT (hazard ratio, 2.53; 95% CI, 1.41–4.53; P = 0.002), prostate-specific antigen (hazard ratio, 1.03; 95% CI, 1.00–1.05; P = 0.037), and Gleason score (>7: hazard ratio, 2.49; 95% CI, 1.25–4.95; P = 0.009). Patients with pathologic 11C-choline uptake in the prostatic bed or in pelvic or retroperitoneal lymph nodes had longer PCa-specific survival (median, 12.1 y; 95% CI, 10.5–13.7 y) in comparison to patients with pathologic tracer uptake in the skeleton (median, 9.9 y; 95% CI, 6.8–13.1 y) (log-rank: χ2 = 6.5, P = 0.010). Two internally validated nomograms predicted 10- and 15-y PCa-specific survival probability with an accuracy of 76% and 74%, respectively. In an ancillary analysis, we also showed that 11C-choline PET/CT predicts PCa-specific survival after PET/CT, with similar statistical power. Conclusion: 11C-choline PET/CT predicts PCa-specific survival in PCa patients treated with radical prostatectomy who develop biochemical failure during androgen-deprivation therapy. If independent or multicenter confirmation of these findings is obtained, 11C-choline PET/CT might be more widely used in the follow-up of PCa patients for tailoring salvage therapy.
In recent years, PET/CT with radiolabeled choline (either 11C-choline or 18F-fluorocholine) has emerged as a sensitive technique for restaging prostate cancer (PCa) patients who experience biochemical failure after radical prostatectomy (1–12). Studies have shown that several factors predict 11C-choline or 18F-fluorocholine PET/CT, including trigger prostate-specific antigen (PSA), PSA doubling time (PSADT) or PSA velocity, pathologic stage at radical prostatectomy, Gleason score, and previous biochemical failure (2,5–8). These results lent support to a growing clinical application of PET/CT with radiolabeled choline, especially in Europe. The convincing results from an increasing number of studies recently led the Food and Drug Administration to approve 11C-choline PET/CT for PCa (13).
A major drawback of most 11C-choline PET/CT clinical studies lies in the poor assessment of the accuracy of the technique. Histologic confirmation has been obtained in no more than 15% of patients in most studies, whereas clinical and imaging criteria have been applied with variable methodologic consistency (1,2,5–8,10,14). False-positives are a concern because increased 11C-choline uptake is not specific for carcinogenesis (15,16). Accuracy assessment will be particularly important for early restaging of disease and to monitor response to therapy.
An indirect way to support the accuracy of 11C-choline PET/CT is to demonstrate that pathologic findings have prognostic implication. The impact on survival in PCa has been demonstrated for bone scintigraphy (17) and PET/CT with 18F-fluorodeoxyglucose (18F-FDG) (18) but not with 11C-choline PET/CT. This study was aimed at assessing whether 11C-choline PET/CT predicts PCa-specific survival in PCa patients who experienced biochemical failure during androgen-deprivation therapy.
MATERIALS AND METHODS
Study Population
This retrospective study included 195 PCa patients who underwent 11C-choline PET/CT for restaging of disease after biochemical failure. Briefly, 2,124 consecutive patients referred to our institution for 11C-choline PET/CT from December 1, 2004, to July 31, 2007, were initially considered for eligibility in this study. Enrollment required histologically confirmed adenocarcinoma of the prostate, primary treatment with radical prostatectomy, rising PSA (PSA > 0.2 ng/mL after previous evidence of nonmeasureable PSA) during androgen-deprivation therapy, clinical and pathologic features of interest for multivariate Cox regression analysis, follow-up information regarding the survival status, and informed consent agreement. Because of the retrospective design of the study, only a minor number of patients (10%) satisfied all inclusion criteria. The exclusion of initially considered patients was due to lack of complete clinical and pathologic information necessary for survival analysis, primary therapy other than prostatectomy, no treatment with antiandrogenic drugs, and refusal to sign informed consent. This single-institution study was approved by the ethical committee of the Scientific Institute San Raffaele Hospital. An informed consent form for the execution of the 11C-choline PET/CT scan and for anonymous publication of disease-related information according to the Declaration of Helsinki was signed by each patient.
Follow-up
Clinical surveillance included PSA determinations and rectal examinations every 3–12 mo. The use of imaging techniques, including computer tomography, ultrasonography, MR, and bone scintigraphy was variable among patients.
The median interval after radical prostatectomy was 8.9 y (95% confidence interval [CI], 1.7–18.9 y), and median follow-up after 11C-choline PET/CT was 4.5 y (95% CI, 0.4–8.5 y). At last follow-up, 84 of 195 (43%) patients had died: 79 of 195 (41%) patients had died of PCa and 5 of 84 (2%) died of causes not related to PCa. PCa-specific survival was chosen as a primary endpoint. PCa-specific death was defined as death in any patient with metastases that showed progression after hormonal therapy without another obvious cause of death (19). Metastases in patients with negative 11C-choline PET/CT findings were documented with different techniques, including bone scintigraphy, contrast-enhanced CT, MR, or subsequent 11C-choline PET/CT. PCa-specific survival was defined as the time between radical prostatectomy and the time of PCa-specific death (19–22). Survival times of patients who were alive at the last follow-up contact were censored.
PET/CT Acquisition
PET/CT studies were acquired by means of 3 integrated PET/CT systems, namely, Discovery LS, Discovery ST, and Discovery STE (GE Healthcare). Patients refrained from drinking and fasted for at least 6 h before 11C-choline PET/CT. A CT scout scan was initially acquired to define the body axial extension (from the pelvis to the base of the skull) to be imaged. After an unenhanced CT scan (90 mAs, 0.8 s per rotation, 140 kV, 4.25-mm reconstructed section thickness) from the pelvis to the base of the skull, five 1-min frames centered on the pelvis were acquired immediately after the injection of 406 ± 48 MBq of 11C-choline using dynamic acquisition. At the end of dynamic imaging, that is, about 5 min after injection, a whole-body PET scan was acquired starting from the pelvis. Images were acquired in 2-dimensional mode (4 min per bed) with the Discovery LS and ST scanners and in 3-dimensional mode (2.5 min per bed) with the Discovery STE scanner. PET images were corrected for random, scatter, and attenuation and reconstructed on a 128 × 128 image matrix using iterative algorithms (8). The axial spatial resolution of the 3 tomographs ranges between 5 and 6 mm (8). A previous study showed no significant differences in the accuracy of the 3 scanners for detection of recurrent disease in PCa patients (8).
Image Interpretation
Image readout was performed on a Xeleris WorkStation (GE Healthcare). 11C-choline PET/CT images were read independently by 2 experienced nuclear medicine physicians. Cases of disagreement were reexamined, and a consensus was reached. A 11C-choline PET/CT scan was called positive in the presence of focal uptake deviating from the physiologic distribution of the tracer independently by semiquantitative uptake values (1,5). For lymph nodes, focal 11C-choline uptake was considered pathologic when greater than background or blood pool independently by lymph node size (5,8). Because the parameter of interest was represented by PCa-specific survival, we made no attempt to validate PET/CT findings versus clinical data or other imaging modalities.
Statistical Analysis
Continuous variables between 2 groups were compared using the t test. The χ2 test was used for categoric variables. The normality of the distribution of continuous variables was assessed with the Kolmogorov–Smirnov test. Nonnormally distributed continuous variables between 2 groups were compared using the Mann–Whitney U test.
PCa-specific survival was estimated using Kaplan–Meier curves. Differences between survival curves of patients with positive 11C-choline PET/CT and negative 11C-choline PET/CT findings were evaluated using the log-rank test. Survival rates and their 95% CIs were also derived from Kaplan–Meier curves.
The association between 11C-choline PET/CT and PCa-specific survival was assessed using Cox proportional hazards regression models. To minimize biases on survival times introduced by therapy successive to 11C-choline PET/CT, additional therapy (radiotherapy or chemotherapy) after 11C-choline PET/CT was included in the analysis. Other variables were age, trigger PSA, pathologic stage, and Gleason score. The effect estimates were expressed as hazard ratios (HRs) with 95% CIs. To assess the additive value of 11C-choline PET/CT in the prediction of survival, a 2-block Cox regression survival analysis was performed. In the first block, all clinical and pathologic variables except 11C-choline PET/CT were included, whereas in the second block 11C-choline PET/CT was added. Results were compared with the Akaike information criterion and χ2 improvement. The proportional hazards assumption was formally tested using interaction terms between independent variables and time. All these interactions had a P value greater than 0.1. Statistical significance was defined as a P value less than 0.05. Survival analysis was performed using a statistical software package (SPSS, version 18; SPSS Inc.).
The coefficients of the covariates included in the Cox regression analysis were used to develop novel nomograms predicting PCa-specific survival probability. The discrimination of the model was quantified using Harrell’s concordance index. One thousand bootstrap resamples were used to internally validate the estimations of the model. Subsequently, the relationship between the nomogram-predicted probabilities and the observed survival rates was graphically explored with calibration plots using the val.prob function at each specific examined time point (R statistical package system; R Foundation for Statistical Computing).
RESULTS
The clinical and pathologic characteristics of the sample are reported in Table 1. 11C-choline PET/CT results were positive in 112 of 195 patients (57%). On an anatomic basis, pathologic 11C-choline uptake was observed in pelvic or retroperitoneal lymph nodes (64/195, 33%), in the skeleton (49/195, 25%), and in the postsurgical prostate bed (36/195, 18%). After 11C-choline PET/CT, radiotherapy or chemotherapy was performed in 23% of patients with negative 11C-choline PET/CT and in 71% of patients with positive 11C-choline PET/CT (P < 0.05). Patients with positive 11C-choline PET/CT had significantly (P < 0.05) higher PSA, more advanced pathologic stage, and shorter PSADT than patients with negative PET/CT (Table 1).
Clinical and Pathologic Characteristics of Whole Sample
In the whole group, the 5-, 10-, and 15-y PCa-specific survival probabilities were, respectively, 88.6% (95% CI, 86.3%–90.9%), 66.0% (95% CI, 62.2%–69.8%), and 30.0% (95% CI, 23.7%–36.3%) (Fig. 1).
Twenty-year actuarial Kaplan–Meier PCa-specific survival probability curve in whole sample. Numbers indicate patients at risk at time tn and number of PCa-specific deaths occurred between tn and tn + 2.
Figure 2 shows Kaplan–Meier PCa-specific survival curves stratified by 11C-choline PET/CT. The median PCa-specific survival was 16.4 y (95% CI, 14.0–18.8 y) in patients with negative 11C-choline PET/CT and 11.2 y (95% CI, 9.8–12.6 y) in patients with positive 11C-choline PET/CT (log-rank: χ2 = 19.3, P < 0001). The 5-, 10-, and 15-y PCa-specific survival probabilities for the 2 groups are reported in Table 2.
Kaplan–Meier PCa-specific survival probability curves in patients with negative 11C-choline PET/CT and in patients with positive 11C-choline PET/CT. Numbers indicate patients at risk at time tn and number of PCa-specific deaths occurred between tn and tn + 2.
PCa-Specific Survival Probabilities (%)
Results of univariate and multivariate Cox regression analysis are shown in Table 3. At multivariate analysis, statistical significance was obtained for 11C-choline PET/CT (HR, 2.53; 95% CI, 1.41–4.53; P = 0.002), PSA (HR, 1.03; 95% CI, 1.00–1.05; P = 0.037), and Gleason score (>7: HR, 2.49; 95% CI, 1.25–4.95; P = 0.009).
Cox Regression Analysis of Factors Predicting PCa-Specific Survival in Whole Sample (n = 195)
In the 2-block survival analysis, the block that additionally included 11C-choline PET/CT yielded a significantly better prediction than the block that included only PSA, age, pathologic stage, Gleason score, and additional therapy (change from previous block: χ2 = 10.8, P = 0.001; Akaike information criterion with and without 11C-choline PET/CT: 267.2 vs. 274.1, respectively).
Cox regression analysis was repeated in patients for whom PSADT was available (n = 76) (Table 4). At bivariate analysis, both 11C-choline PET/CT (HR, 2.75; 95% CI, 1.04–7.32; P = 0.042) and PSADT (HR, 0.88; 95% CI, 0.79–0.98; P = 0.029) attained statistical significance.
Cox Regression Analysis of Factors Predicting PCa-Specific Survival in Patients with PSADT (n = 76)
Patients with pathologic 11C-choline uptake in the prostatic bed or in pelvic or retroperitoneal lymph nodes but no evidence of pathologic bone uptake had shorter PCa-specific survival (median, 12.1 y; 95% CI, 10.5–13.7 y) in comparison to patients with negative PET/CT (log-rank: χ2 = 8.9, P = 0.003) but longer PCa-specific survival in comparison to patients with pathologic tracer uptake in the skeleton (median, 9.9 y; 95% CI, 6.8–13.1 y) (log-rank: χ2 = 6.5, P = 0.011) (Fig. 3). Patients with pathologic tracer uptake in the skeleton had shorter PCa-specific survival than patients with negative PET/CT (log-rank: χ2 = 27.8, P < 0.001). PCa-specific survival probabilities for these groups are reported in Table 5. We also assessed survival according to the TNM classification of the American Joint Committee on Cancer (23), assuming 11C-choline PET/CT as a gold standard for identification of disease. Contrasting patients with pathologic 11C-choline uptake in the skeleton (M1b) or in retroperitoneal lymph nodes (M1a) to patients with pathologic 11C-choline uptake in pelvic lymph nodes (N1 M0) or in the prostate bed (N0) provided similar results, even though group differences in survival, as expected, were somehow increased (Table 5).
Kaplan–Meier PCa-specific survival probability curves in patients with negative 11C-choline PET/CT (PET/CT−), in patients with 11C-choline PET/CT suggestive of local recurrence or lymph node disease (PET/CT+ LR/Lfn), and in patients with 11C-choline PET/CT suggestive of bone metastases (PET/CT+ bone). Numbers indicate patients at risk at time tn and number of PCa-specific deaths occurred between tn and tn + 2.
PCa-Specific Survival Probabilities (%) in Patients With Pathologic 11C-Choline Uptake and in Patients Without (M0) and With (M1) Distant Metastases
To assess the sensitivity of our results to the definition of PCa-specific survival, an ancillary Cox regression analysis was performed in which PCa-specific survival was computed as the interval between the date of 11C-choline PET/CT and the date of PCa-specific death. At multivariate analysis, statistical significance was obtained for 11C-choline PET/CT (HR, 3.16; 95% CI, 1.72–5.78; P < 0.001), age (HR, 1.06; 95% CI, 1.02–1.10; P = 0.002), PSA (HR, 1.05; 95% CI, 1.02–1.07; P < 0.001), and pathologic stage (any T pN1: HR, 1.85; 95% CI, 1.02–3.38; P = 0.043) (Table 6). Median PCa-specific survival after 11C-choline PET/CT was 7.9 y (95% CI, 5.3–10.5 y) in patients with negative 11C-choline PET/CT and 4.1 y (95% CI, 2.5–5.6 y) in patients with positive 11C-choline PET/CT (log-rank: χ2 = 30.9, P < 0.001). The 5-y PCa-specific survival probability after 11C-choline PET/CT was 82.9% (95% CI, 78.6%–87.2%) in patients with negative 11C-choline PET/CT and 44.7% (95% CI, 39.5%–49.9%) in patients with positive 11C-choline PET/CT. At bivariate Cox regression analysis in patients with PSADT (n = 76) (Table 7), 11C-choline PET/CT (HR, 4.51; 95% CI, 1.68–12.11; P = 0.003) but not PSADT (HR, 0.93; 95% CI, 0.84–1.03; P = 0.178) attained statistical significance.
Cox Regression Analysis of Factors Predicting PCa-Specific Survival (Calculated from Time of 11C-Choline PET/CT) in Whole Sample (n = 195)
Cox Regression Analysis of Factors Predicting PCa-Specific Survival (Calculated from Time of 11C-Choline PET/CT) in Patients with PSADT (n = 76)
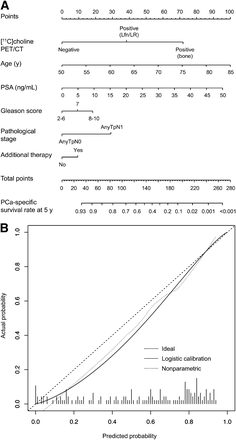
The nomograms predicting 10- and 15-y PCa-specific survival probability after radical prostatectomy and 5-y PCa-specific survival probability after 11C-choline PET/CT together with their calibration plots are shown in Figures 4–6. The internally validated discriminations of the model were, respectively, 76%, 74%, and 73%. The calibration plots between nomogram-predicted-probability PCa-specific survival rate and observed surviving fraction indicate good prediction throughout the range of predicted probabilities. However, the nomogram that predicts the 15-y PCa-specific probability tended to overestimate the real rate of survival (Fig. 5B).
Nomogram (A) and calibration factor (B) predicting 10-y PCa-specific survival probability (calculated from radical prostatectomy). Perfect prediction would correspond to diagonal 45° broken line. Dotted line indicates actual nomogram performance. Expected performance on future data is represented through solid line.
Nomogram (A) and calibration factor (B) predicting 15-y PCa-specific survival probability (calculated from radical prostatectomy).
Nomogram (A) and calibration factor (B) predicting 5-y PCa-specific survival probability (calculated from 11C-choline PET/CT).
DISCUSSION
Over the last years, there has been growing evidence that PET/CT with radiolabeled choline may be useful for restaging PCa patients with biochemical failure after radical prostatectomy (1–12). For this reason, the Food and Drug Administration recently approved 11C-choline PET/CT for PCa (13). However, in most recent guidelines of international oncologic and urologic societies 11C-choline PET/CT is not recommended either for restaging PCa patients with biochemical failure or for evaluation of response in clinical trials (24–26), and the role of 11C-choline PET/CT was recently debated between members of the European Association of Urology and of the European Association of Nuclear Medicine and Molecular Imaging (26,27).
In our opinion, there are 3 main reasons that give support to the limited acceptance of 11C-choline PET/CT among clinicians worldwide. First, most clinical studies with PET/CT and either 11C-choline or 18F-fluorocholine offered only poor validation of PET/CT findings. Histologic confirmation has been obtained in no more than 15% of patients of most 11C-choline PET/CT studies, whereas clinical and imaging criteria have been applied with variable methodologic consistency (1,2,5–8,10,14). Methodologic problems related to this issue have been previously discussed (6–8,10,14) and represented a motivation for the present study. False-positive 11C-choline PET/CT findings are a concern because increased 11C-choline uptake is not specific for carcinogenesis (15,16). In studies with largest samples, false-positives were reported in about 15% of patients who underwent salvage lymphadenectomy (28,29). On the other hand, the limited spatial resolution of PET/CT significantly limits the detection of microscopic disease and underestimates the extent of lymph node disease, as assessed by histologic analysis (28,29).
The second reason is that change of management has not been the focus of most studies. Soyka et al. did report change of management using PET/CT with 18F-fluorocholine in 48% of PCa patients (30). However, in this study change of management was assessed retrospectively a minimum of 14 mo after PET/CT.
The third and possibly most important reason is that prognostic implications of 11C-choline PET/CT have been so far scarcely investigated. This is due to the fact that most studies were cross-sectional and had small sample size and limited follow-up. To the best of our knowledge, only Breeuwsma et al. preliminary reported on this issue (31). In this study that included 64 patients, 10 patients died during follow-up, of which 5 patients died due to metastasized disease. For this reason, the study was not powered to detect significant differences in PCa-specific survival. Kaplan–Meier curves did show shorter overall survival in patients with positive 11C-choline, but median overall survival was not achieved (31). Bone scintigraphy and PET/CT with 18F-FDG are currently considered in the nuclear medicine community less sensitive or less accurate than 11C-choline PET/CT for restaging PCa patients after biochemical failure (1,7,10,32,33). However, previous studies showed that bone scintigraphy (17) and PET/CT with 18F-FDG (18) predict survival in PCa patients.
In the current study, we performed survival analysis in PCa patients who developed biochemical failure during androgen-deprivation therapy. These patients are at a higher risk of PCa-specific mortality in comparison to androgen-sensitive patients (25), and they also have a higher likelihood of positive 11C-choline PET/CT in comparison to patients who are hormone-naïve (8,34). We followed up the present cohort for a median interval of 4.5 y (95% CI, 0.4–8.5 y) after 11C-choline PET/CT. The median PCa-specific survival (calculated from radical prostatectomy) was 16.4 y (95% CI, 14.0–18.8 y) in patients with negative 11C-choline PET/CT and 11.2 y (95% CI, 9.8–12.6 y) in patients with positive 11C-choline PET/CT. The median PCa-specific survival after 11C-choline PET/CT was 7.9 y (95% CI, 5.3–10.5 y) in patients with negative 11C-choline PET/CT and 4.1 y (95% CI, 2.5–5.6 y) in patients with positive 11C-choline PET/CT.
Several clinical and pathologic factors have been shown in larger studies to predict PCa-specific mortality, including Gleason score, pathologic stage, PSA, PSADT, and PSA velocity (19–22). In the current study, when 11C-choline PET/CT was added to the regression model including clinical and pathologic factors the stratification of PCa-specific death risk significantly improved.
Patients with 11C-choline PET/CT findings suggestive of skeletal metastases had shorter survival in comparison to patients with PET/CT findings suggestive of lymph node metastases or local recurrence (Fig. 3; Table 5) consistent with the observation that skeletal metastases represent the primary source of morbidity and mortality in PCa patients (17,35). Analysis according to the American Joint Committee on Cancer staging system provided similar results (Table 5). In patients experiencing biochemical failure, it is important to identify the site of recurrence to stratify the risk of death. Patients at high risk of death are those who require more aggressive treatment, whereas overtreatment could be reduced in patients who are less likely to rapidly progress (21). If images suggest pelvic lymph node disease, pelvic lymph nodes can be included in the radiation plan (36). Combined treatment of prostate bed and lymph nodes might improve progression-free survival (37). Our results suggest that 11C-choline PET/CT might be a suitable technique to identify patients with different prognosis for potential stratification for salvage lymphadenectomy or radiotherapy in oncologic trials.
PCa-specific survival was traditionally computed as the interval between radical prostatectomy and PCa-specific death. This was motivated by the fact that 11C-choline PET/CT was performed neither at a fixed trigger PSA value nor at the fixed time after radical prostatectomy. If so calculated, survival time is standardized for all patients and it is not influenced by several biases, including modality of referral to 11C-choline PET/CT. On the other hand, computation of survival time starting from the date of 11C-choline PET/CT may be more practical information for patient counseling and therapy decision. Comparison of Table 3 with Table 6 and Table 4 with Table 7 does indicate that the predictive role of most chosen variables is influenced by the definition of survival time. For example, Gleason score and PSADT attained statistical significance only if survival time is computed from radical prostatectomy, whereas the predictive power of age, PSA, and pathologic stage is more robust when survival is computed starting from the date of PET/CT. This differential sensitivity may have methodologic and biologic explanations. For example, Gleason score at radical prostatectomy may not accurately reflect Gleason score at recurrence (38). High PSA measured at the time of PET/CT is intuitively more related to residual survival time rather than to survival from radical prostatectomy. On the contrary, 11C-choline PET/CT was highly significant at multivariate analysis independent of the method chosen for the calculation of survival time. Therefore, among the considered variables 11C-choline PET/CT appears to be the most powerful and reliable predictor of PCa-specific survival. This means that 11C-choline PET/CT can be used to accurately predict PCa-specific survival from radical prostatectomy and PCa-specific survival after PET/CT.
Fast PSA kinetics, expressed either as PSADT or PSA velocity, has been often reported as the single most important factor predicting survival in PCa (19–21). Because of the retrospective design of the study, PSADT was available only for 39% of patients, so that PSADT was not included in the main analysis. In this small sample, 11C-choline PET/CT appears to be superior to PSADT for the prediction of PCa-specific survival after 11C-choline PET/CT (Table 7).
Potential limitations of this study need to be considered. This study was designed retrospectively. 11C-choline PET/CT was defined as positive only on the basis of tracer uptake, independently by radiologic findings. Time-dependent changes in bone uptake of radiolabeled choline as a function of changes in Hounsfield units of bone metastases are currently under investigation (14). Finally, therapeutic interventions, which certainly took place after 11C-choline PET/CT, were not further considered in the framework of this data analysis although they might have added relevant information for the raised question. Future prospective studies should assess the comparative value of 11C-choline PET/CT versus conventional imaging for management of PCa patients and prediction of survival.
CONCLUSION
The present observational study indicates that positive 11C-choline PET/CT in PCa patients who develop biochemical failure during androgen-deprivation therapy predicts shorter PCa-specific survival. The prediction of PCa-specific survival is robust and independent of the method of calculation of survival time. Patients with 11C-choline PET/CT scans suggesting local recurrence or lymph node disease had longer PCa-specific survival than patients with presumed bone metastases. If independent or multicenter confirmation of these findings is obtained, 11C-choline PET/CT might be more widely used in the follow-up of PCa patients for tailoring salvage therapy.
DISCLOSURE
The costs of publication of this article were defrayed in part by the payment of page charges. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734. No potential conflict of interest relevant to this article was reported.
Footnotes
Published online Jan. 9, 2014.
- © 2014 by the Society of Nuclear Medicine and Molecular Imaging, Inc.
REFERENCES
- Received for publication March 27, 2013.
- Accepted for publication August 2, 2013.