Abstract
The prognostic value of interim PET or PET/CT performed after 1–4 cycles of chemotherapy has been widely confirmed in Hodgkin lymphoma and diffuse large B-cell lymphoma but remains unknown in T-cell and natural killer (T/NK) cell lymphomas. Therefore, our aim was to investigate the prognostic value of interim and posttherapy PET/CT in T/NK-cell lymphomas. Methods: A retrospective analysis was conducted on data from 88 patients with newly diagnosed T/NK-cell lymphoma who underwent interim (after 1–4 cycles of chemotherapy, n = 62) or posttherapy PET/CT (after the completion of first-line therapy, n = 47). Interim and posttherapy PET/CT status (positive vs. negative) was visually interpreted according to criteria of the International Harmonization Project, and PET/CT status was assessed for its ability to predict progression-free survival (PFS) and overall survival (OS). Results: Interim PET/CT results were negative in 17 of 62 (27.4%) cases, and posttherapy PET/CT results were negative in 29 of 47 (61.7%) cases. The 2-y PFS and OS rates were 71.9% and 80.2%, respectively, in patients with negative results at interim PET/CT versus 20.5% and 46.9%, respectively, in patients with positive results (P < 0.001 and P = 0.022, respectively). The 2-y PFS and OS rates were 57.8% and 78.0%, respectively, in patients with negative results on posttherapy PET/CT versus 0% and 20.4%, respectively, in patients with positive results (P < 0.001 and P = 0.003, respectively). Bivariate analysis showed that interim PET/CT status and posttherapy PET/CT status remain independent predictors of PFS and OS after controlling for the score on the Prognostic Index for Peripheral T-Cell Lymphoma, Unspecified. Conclusion: Both interim PET/CT status and posttherapy PET/CT status are independent predictors of PFS and OS in T/NK-cell lymphomas.
Mature T-cell and natural killer (NK) cell lymphomas are rare and heterogeneous, accounting for about 5%–10% of non-Hodgkin lymphomas (NHL) in Western countries (1), and they are less common in Western countries than in Asia, where they account for 15%–20% of NHL (1,2). The prognosis of T/NK-cell lymphomas is poorer than that of B-cell NHL. To date, there is no consensual standard treatment strategy for T/NK-cell lymphomas. After conventional chemotherapy with or without radiotherapy, patients with T/NK-cell lymphoma subtypes other than anaplastic lymphoma kinase (ALK)–positive anaplastic large cell lymphoma (ALCL) and primary cutaneous T/NK-cell lymphoma have a 5-y overall survival (OS) rate of between 7% and 49% (1).
18F-FDG PET is a functional imaging test that has been widely used for assessing initial staging and monitoring response to treatment in both Hodgkin lymphoma (HL) and NHL (3). Recent studies have also confirmed the prognostic value of interim PET or PET/CT performed after 1–4 cycles of chemotherapy for HL and diffuse large B-cell lymphoma (DLBCL) (4–8). Furthermore, multiple studies demonstrate that PET is both sensitive and specific for initial staging in T/NK-cell lymphomas (9–13). However, the prognostic role of interim and posttherapy PET/CT in T/NK-cell lymphomas has not been elucidated. Furthermore, the poor prognosis and extremely significant heterogeneity of T/NK-cell lymphomas emphasizes the need for more effective prognostic factors or tools to identify those patients most likely to benefit from therapy. We investigated the prognostic value of interim and posttherapy PET/CT in T/NK-cell lymphomas in a retrospective single-center study.
MATERIALS AND METHODS
Patient Selection
Between December 2004 and June 2011, we included 88 consecutive adult patients with newly diagnosed and histologically proven T/NK-cell lymphomas according to the classification of the World Health Organization (14). To be included, patients had to have undergone interim (after 1–4 cycles of chemotherapy) whole-body PET/CT, posttherapy (after first-line treatment) whole-body PET/CT, or both. Baseline PET/CT was optional. Patients with ALK-positive ALCL, patients with primary cutaneous T/NK-cell lymphomas and children and adolescents with lymphomas were excluded because of their favorable prognosis or receipt of distinct treatments. At baseline and after completion of treatment, all patients underwent the following standard evaluations: a complete history and physical examination; blood cell counts; conventional biochemical profile; bone marrow aspiration and biopsy; CT or MR imaging of the head and neck (if necessary); and CT scans of the thorax, abdomen, and pelvis. Baseline, interim, and posttherapy CT and PET/CT were assessed according to the revised International Workshop Criteria (15). This study protocol was approved by the Institutional Review Board of the National Cancer Institute and by the ethics committees of Sun Yat-Sen University Cancer Center. The study was conducted in accordance with the Declaration of Helsinki and the institutional guidelines of the local ethics committee. All patients signed a written informed consent form before treatment.
PET/CT Scan Protocol
PET/CT was performed at baseline (in some patients), after 1–4 cycles of chemotherapy (interim PET/CT), or after completion of first-line treatment (chemotherapy, radiotherapy, and autologous bone marrow transplantation when performed as first-line therapy [posttherapy PET/CT]). In some patients, both interim and posttherapy PET/CT was performed. For all patients, whole-body 18F FDG PET/CT was performed with a combined PET/CT scanner (Discovery ST, with a 16-slice CT component; GE Healthcare Bio-Sciences Corp.). After 6 h of fasting (blood glucose < 8 mmol/L), an intravenous injection of 215–522 MBq of 18F-FDG was administered, and after approximately 60 min of resting, a whole-body CT scan and PET scan extending from the head to the mid-thigh level were obtained with 6–8 bed positions. A CT scan was obtained initially with a voltage of 140 kV, a current intensity of 150–160 mA, a tube rotation of 0.8 s, and a section thickness of 5 mm, without an oral or intravenous contrast agent. The CT acquisition data were used for attenuation correction, and corrected PET images were reconstructed using a standard iterative algorithm (ordered-subset expectation maximization). The acquired images from PET and CT were sent to an Entegra or Xeleris (GE Healthcare) workstation for image registration and fusion.
Image Analysis
All fused PET/CT images were visually interpreted by a consensus of 2 experienced nuclear medicine physicians who were masked to the clinical information on patients. According to the criteria of the International Harmonization Project (16), a PET/CT scan is defined as positive if there is focal or diffuse 18F-FDG uptake greater than the background level of uptake or if the liver in a location incompatible with normal anatomy or physiology, and a PET/CT scan is defined as negative if no pathologic 18F-FDG uptake appears at any site. Whenever possible, 18F-FDG uptake was interpreted in light of the baseline PET/CT scan. Maximum standardized uptake value (SUVmax) was calculated as follows: SUVmax = decay-corrected activity in tissue (MBq/mL)/(injected dose of 18F-FDG [MBq]/lean body mass [kg]) (7). The percentage of SUVmax reduction from baseline to interim PET/CT (ΔSUVmax) was calculated as follows: ΔSUVmax = 100 × (SUVmax [baseline PET/CT] − SUVmax [interim PET/CT])/SUVmax (baseline PET/CT) (7).
Statistical Methods
Progression-free survival (PFS) was defined as the interval between the date of diagnosis and the date of first relapse, progression, death from any cause, or last follow-up. OS was defined from the day of diagnosis until the time of death from any cause or last follow-up. The relationships of interim and posttherapy PET/CT status with clinical variables were analyzed by χ2 tests. Comparisons of average baseline SUVmax among 5 subtypes of lymphoma and comparison of average ΔSUVmax between PET/CT-negative and PET/CT-positive groups at interim were performed using 1-way ANOVA with a Brown–Forsythe F test. The log-rank test and Kaplan–Meier method were used for univariate survival analysis. Because of the small number of patients in each group, a bivariate Cox proportional hazards model analysis was performed to determine whether positive interim or posttherapy PET/CT was associated significantly with PFS and OS after adjusting for the score on the Prognostic Index for Peripheral T-cell Lymphoma, Unspecified (PIT). A 2-tailed P value of less than 0.05 was considered to be statistically significant. The statistical software package SPSS (version 16.0; SPSS) was used for statistical calculations.
RESULTS
Patient Characteristics and Outcome
Eighty-eight patients with T/NK-cell lymphomas (63 men, 25 women; median age, 42 y [age range, 18–76 y]) were included in the present study. The main characteristics and first-line chemotherapy regimens for patients are summarized in Table 1. Induction chemotherapy usually consisted of 2–8 cycles (median, 6 cycles) of 1 of the following 4 regimens: CHOP, EPOCH, alternating triple therapy (CHOP-B, IMVP-16, and DHAP), or GEMOX + L-asp (Table 1) (CHOP is cyclophosphamide, doxorubicin, vincristine, and prednisone; EPOCH is etoposide, doxorubicin, vincristine, cyclophosphamide, and prednisone; IMVP-16 is ifosfamide, methotrexate, and etoposide; DHAP is dexamethasone, cisplatin, and cytarabine; GEMOX is gemcitabine and oxaliplatin.) Forty-two (47.7%) patients received radiotherapy during or after completion of first-line chemotherapy. Baseline clinical features and first-line chemotherapy regimens were compared according to the status of the interim and posttherapy PET/CT (positive vs. negative). No significant between-group difference was found for any characteristic (Table 1). During a median follow-up of 19.5 mo (range, 2–82 mo), 37 patients (42%) died. The median OS for all 88 cases was 11 mo. The 2-y PFS and OS rates for all 88 patients were 35.5% and 55%, respectively.
Patient Characteristics and Treatment
SUVmax at Baseline
Baseline PET/CT scans were acquired in 53 of 88 patients (60.2%) and showed a positive result in 51 cases (96.2%). The histologic subtypes of the 2 patients with a negative result were extranodal NK/T-cell lymphoma (nasal involvement) and peripheral T-cell lymphoma, not otherwise specified (nodal involvement), respectively. The average and median SUVmax at baseline PET of different subtypes of T/NK-cell lymphoma are listed in Table 2. The mean SUVmax ± SD of ALK-negative anaplastic large cell lymphoma (ALCL) was significantly higher than that of extranodal NK/T-cell lymphoma (ENKL) (P = 0.002); peripheral T-cell lymphoma, not otherwise specified (PTCL-NOS) (P = 0.007); enteropathy-associated T-cell lymphoma (EATL) (P = 0.020); and angioimmunoblastic T-cell lymphoma (AITL) (P = 0.047). There was no significant difference in mean SUVmax ± SD among ENKL, PTCL-NOS, EATL, and AITL (all P > 0.05).
Baseline SUVmax of Different Subtype of T/NK-cell Lymphomas
Interim PET/CT Analysis
Given the high sensitivity (96.2%) of PET/CT scans at diagnosis, outcome analysis was performed on all 88 patients, regardless of whether they had undergone baseline scanning. Of the 88 patients, 62 underwent interim PET/CT (performed after 1–4 cycles of chemotherapy [median, 3 cycles]). The median time from completion of the previous course to performance of the interim PET/CT scan was 20 d (range, 7–39 d). Interim PET/CT scans were interpreted as negative in 17 patients (27.4%) and positive in 45 patients (72.6%). Positive results on interim PET/CT were significantly correlated with inferior PFS (P < 0.001; Fig. 1A) and OS (P = 0.022, Fig. 1B). The 2-y PFS and OS rates were 71.9% and 80.2%, respectively, in patients with negative results on interim PET/CT versus 20.5% and 46.9%, respectively, in patients with positive results. The prognostic value of interim PET/CT was also analyzed according to histologic subtypes (ENKL vs. non-ENKL T-cell lymphomas [including PTCL-NOS, ALK-negative ALCL, AITL, and EATL]). In ENKL (n = 34), there was a significant between-group difference in PFS (P = 0.013, Fig. 2A) but not in OS (P = 0.278, Fig. 2B). However, in non-ENKL T-cell lymphomas (n = 28), positive results on interim PET/CT correlated significantly with inferior PFS (P = 0.002; Fig. 2C) and OS (P = 0.026, Fig. 2D). Positive and negative predictive values (PPV and NPV, respectively) and accuracy in predicting PFS and OS in 62 patients who underwent interim PET/CT are listed in Table 3. Of 45 interim PET/CT‐positive patients, 35 (77.8%) showed treatment failure (progression or relapse), and 23 (51.1%) died during the follow-up.
Outcome Prediction by Interim and Posttherapy PET/CT Analysis
Survival outcomes according to interim PET/CT status: PFS in T/NK-cell lymphomas (A) and OS in T/NK-cell lymphomas (B).
Survival outcomes according to histologic subtypes at interim PET/CT analysis: PFS in ENKL (A), OS in ENKL (B), PFS in non-ENKL T-cell lymphomas (C), and OS in non-ENKL T-cell lymphomas (D).
Of the 53 patients with baseline PET/CT, 45 underwent interim PET/CT. The percentages of SUVmax reduction from baseline PET/CT to interim PET/CT (ΔSUVmax) were calculated for each patient in both PET/CT-negative and PET/CT-positive groups at interim. Results showed that the average ΔSUVmax of patients in the PET/CT-negative group (n = 13) (mean ± SD, 81.5% ± 8.9%) was significantly higher than those in the PET/CT-positive group (n = 32) (mean ± SD, 23.3% ± 67.1%) (P = 0.003).
Posttherapy PET/CT Analysis
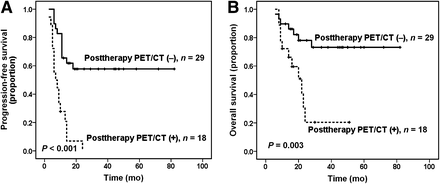
Forty-seven patients underwent posttherapy PET/CT within a median interval of 22 d (range, 11–90 d) after all planned first-line treatment. Posttherapy PET/CT scans were interpreted as negative in 29 patients (61.7%) and positive in 18 patients (38.3%). Similar to interim PET/CT, positive results on posttherapy PET/CT were also significantly associated with shorter PFS (P < 0.001; Fig. 3A) and OS (P = 0.003, Fig. 3B). The 2-y PFS and OS rates were 57.8% and 78.0%, respectively, in patients with negative results on posttherapy PET/CT versus 0% and 20.4%, respectively, in patients with positive results. Again in ENKL (n = 17), there was a significant between-group difference in PFS (P = 0.013, Fig. 4A) but not in OS (P = 0.238, Fig. 4B). However, in non-ENKL T-cell lymphomas (n = 30), the positive posttherapy PET/CT result was significantly correlated with both inferior PFS and OS (P < 0.001 and P = 0.006, respectively; Figs. 4C and 4D). The PPV, NPV, and accuracy of posttherapy PET/CT in predicting PFS and OS are listed in Table 3.
Survival outcomes according to posttherapy PET/CT status: PFS in T/NK-cell lymphomas (A) and OS in T/NK-cell lymphomas (B).
Survival outcomes according to histologic subtypes at posttherapy PET/CT analysis: PFS in ENKL (A), OS in ENKL (B), PFS in non-ENKL T-cell lymphomas (C), and OS in non-ENKL T-cell lymphomas (D).
Of the 47 patients with posttherapy PET/CT, 21 also underwent interim PET/CT. For the 21 patients who underwent PET/CT twice, 8 (38.1%) had negative results and 13 (61.9%) positive results at interim PET/CT, and 11 (52.4%) had negative results and 10 (47.6%) positive results at posttherapy PET/CT. In the 8 patients with a negative interim PET/CT result, 7 (87.5%) continued to have negative results at posttherapy PET/CT, whereas the results for 1 (12.5%) changed to positive at posttherapy PET/CT. Similarly, 9 of the 13 (69.2%) interim PET/CT‐positive patients remained positive at posttherapy PET/CT, whereas 4 (30.8%) converted to a negative status at posttherapy PET/CT. Positive interim PET/CT scans were significantly correlated with positive posttherapy PET/CT scans (P = 0.01).
Response at Interim and Posttherapy Evaluation
At the interim evaluation, a complete response (CR) was achieved in 19 of 62 patients (30.6%). The concordance between clinical CR by CT or MR imaging criteria and interim PET negativity was 89.5%: 2 patients—although in CR without any residual masses—had false-positive results at interim PET. At the posttherapy evaluation, CR was achieved in 31 of 47 patients (66%). The concordance between clinical CR and posttherapy PET negativity was 93.5%. However, 2 patients—although in CR without any residual masses—had false-positive results at posttherapy PET. Moreover, 5 of 17 patients with negative interim PET results and 3 of 29 patients with negative posttherapy PET results had false-positive residual masses (mass > 1.5 cm) on interim and posttherapy CT or MR imaging, respectively. The 3 patients with residual masses on posttherapy CT or MR imaging were in continuous CR until the time of analysis.
Uni- and Bivariate Analyses for PFS and OS
Table 4 shows the results of univariate analysis of clinical variables considered as predictors of PFS and OS. Among patients who underwent interim PET/CT, univariate analysis revealed that a PIT score of 2 or greater and positive results at interim PET/CT were significantly unfavorable prognostic factors for both PFS (P = 0.023 and P < 0.001, respectively) and OS (P = 0.003 and 0.022, respectively) (Table 4). For those patients who underwent posttherapy PET/CT, univariate analysis showed that positive results at posttherapy PET/CT were the only significant predictor of unfavorable PFS (P < 0.001), whereas the significant predictors of unfavorable OS included age more than 60 y, elevated lactate dehydrogenase, a PIT score of 2 or more, and a positive posttherapy PET/CT result (all P < 0.05). Given the small number of patients in each group, 2 independent bivariate Cox model analyses were performed to properly evaluate the prognostic role of interim and posttherapy PET/CT results for PFS and OS after controlling for a PIT score of 2 or more. For those patients who underwent interim PET/CT, bivariate analysis demonstrated that positive interim PET/CT remained a significant predictor of decreased PFS (P = 0.001) and OS (P = 0.048), and the PIT score remained an independent prognostic factor for OS (P = 0.006) but not PFS (P = 0.065) (Table 5). Similarly, for those patients who underwent posttherapy PET/CT, bivariate analysis showed that positive results at posttherapy PET/CT remained a highly significant independent predictor of inferior PFS (P < 0.001) and OS (P = 0.009), and PIT score retained its prognostic value for OS (P = 0.013) but not PFS (P = 0.725) (Table 5).
Univariate Analysis of Factors Predictive of PFS and OS
Bivariate Analysis of Factors Predictive of PFS and OS
DISCUSSION
The prognostic role of early 18F-FDG PET in DLBCL and HL has been widely confirmed by numerous studies (4–8); however, studies focusing on the prognostic value of interim or posttherapy 18F-FDG PET in T/NK-cell lymphomas are indeed rare and the results are contradictory (17–19). In the present study, the prognostic value of interim and posttherapy PET/CT was assessed in a series of patients with T/NK-cell lymphomas.
In the present study, a positive interim PET/CT result was found to be significantly correlated with inferior PFS and OS in T/NK-cell lymphomas, confirming the findings of Choi et al. but not the findings of Cahu et al. and Pro et al. (17–19). Some technical differences studies may explain this discrepancy. First, the studies by Pro et al. and Cahu et al. used PET as the method for interim evaluation, whereas our study and the study of Choi et al. used PET/CT, which has been shown to increase the sensitivity and specificity of PET and improve the accuracy of monitoring of therapeutic responses (20). Consistent with previous findings (20), we discovered that there was a relatively high incidence of false-positive interim and posttherapy PET scans (4.4% and 11.1%, respectively). Similarly, interim and posttherapy CT or MR imaging also showed a high incidence of false-positive residual masses (10.4% and 15.8%, respectively). Moreover, because the reference background of the criteria of the International Harmonization Project used in current study depends on the size of the residual mass (16), measurement of lesions by CT can facilitate the accurate selection of reference background. Taken together, PET/CT may be superior to PET or CT/MR imaging alone for interim and posttherapy evaluation. Second, patients in the study by Cahu et al. were recruited from 5 independent institutions with different PET systems and protocols, possibly leading to heterogeneity in the results of PET. In addition, the sample size in our study was larger (n = 62 vs. n ≤ 35) (17–19). More important, our study showed a higher PPV of interim PET/CT (77.9% vs. 39%), meaning a high incidence of relapse or progression among patients with positive results at interim PET/CT. It is thus conceivable that patients with positive interim results should be considered candidates for an intensive therapeutic strategy to improve their clinical outcome.
Despite the limitations of PET, promising evidence shows that PET has greater value than CT or MR imaging in terms of initial staging, monitoring of response to therapy, and detection of disease recurrence in HL and NHL (3–13,20). Furthermore, PET has also been incorporated into the response criteria for lymphoma as revised by the International Harmonization Project on Lymphoma (15). In the present study, 5 patients with negative results at interim PET and 3 patients with a negative results at posttherapy PET demonstrated residual masses by interim and posttherapy CT or MR imaging, respectively. One of the potential explanations for this is that metabolic changes occur before morphologic tumor changes. Given that all 3 patients with residual masses by posttherapy CT or MR imaging are alive without evidence of disease, PET shows the efficient ability to identify metabolic activity in residual masses. More important, this observation may have important clinical implications for optimization of therapy because unnecessary, excessive, or intensive salvage treatment may be avoided for patients with a residual mass on CT or MR imaging but truly negative PET results.
Numerous previous studies have revealed that a quantitative approach based on the percentage ΔSUVmax between baseline PET and interim PET may be superior or equivalent to visual analysis in predicting outcome in DLBCL (7,8,21,22). Lin et al. and Casasnovas et al. (7,21) proposed that ΔSUVmax (between baseline PET and PET after 2 cycles of chemotherapy) analysis is a better predictor of outcome than visual analysis in DLBCL. Moreover, 2 more recent studies showed that ΔSUVmax (after 2 or 4 cycles of chemotherapy) analysis and visual analysis are equivalent predictors of outcome in DLBCL (8,22). Quantitative assessment is probably a more objective and effective way to interpret PET results than visual analysis. However, because the optimal cutoff of ΔSUVmax varied between studies (65.7% or 66% after 2 cycles of chemotherapy and 70% or 72.9% after 4 cycles of chemotherapy), the prognostic value of ΔSUVmax-based methods still needs to be validated by further prospective studies in homogeneous populations and using internationally validated reporting criteria. In the present study, because interim PET/CT was performed at different time points (after 1–4 cycles of chemotherapy), ΔSUVmax analysis could not be performed.
In the present study, the status of posttherapy PET/CT was a reliable independent predictor of PFS and OS in T/NK-cell lymphomas. This result is inconsistent with that of Cahu et al., who found no significant difference in PFS or OS between T/NK-cell lymphoma patients with positive results at posttherapy 18F-FDG PET and those with negative results (19). The potential reasons for the difference between our 2 studies are as described above for interim PET/CT. Moreover, in our study, patients with positive results at posttherapy PET/CT had a high rate of relapse or progression (88.9%), suggesting that after the completion of first-line induction therapy they may need to receive further consolidated therapy such as autologous bone marrow transplantation, allogeneic stem cell transplantation, or other targeted therapy (23–25). But such therapies remain controversial and need more prospective studies to validate their efficacy.
Similar to previous retrospective studies, our study had several limitations, including the relatively small size of our cohort, histologic inhomogeneity in the tumors, variation in first-line chemotherapy regimens, variation in the interval between completion of chemotherapy (after 1–4 cycles) and performance of interim PET/CT, and a limited number of patients with baseline PET/CT (only 60.2%). Patients with some special T/NK-cell lymphomas subtypes such as ALK-positive ALCL, primary cutaneous T/NK-cell lymphomas, and lymphomas in childhood and adolescence were excluded because the prognosis for these patients was favorable (1), 18F-FDG avidity of the tumor was variable (9,26,27), and treatment strategies were distinct. Furthermore, no significant between-group difference in chemotherapy regimen distribution was found according to the status of interim and posttherapy PET/CT. These decrease the histologic and therapeutic heterogeneity to some extent. Moreover, our subgroup analysis according to histologic classification (ENKL vs. non-ENKL T-cell lymphomas) also demonstrated that both interim and posttherapy PET/CT results remain significant predictors for PFS in ENKL and significant predictors for PFS and OS in non-ENKL T-cell lymphomas. Several reports reveal that a baseline PET scan may facilitate the interpretation of interim and posttherapy PET results (16,28). In our study, although baseline PET/CT was available for only 72.6% and 60% of patients undergoing interim or posttherapy PET/CT, respectively, the sensitivity was high (95.6% and 100%, respectively). Given the high sensitivity of baseline PET/CT in detecting T/NK-cell lymphomas in the present and previous studies (9–13), outcome analysis was performed on all patients, regardless of whether a baseline PET/CT scan had been obtained.
CONCLUSION
Our study indicates that interim and posttherapy PET/CT results are 2 independent predicators of PFS and OS in T/NK-cell lymphomas. In addition, our data also imply that patients with positive results at interim or posttherapy PET/CT should be considered candidates for an intensive therapeutic strategy to improve their clinical outcome. Large prospective studies of patients with tumors of a homogeneous histologic subtype treated with a uniform protocol and evaluated on the basis of standardized criteria are warranted to evaluate the prognostic value of interim and posttherapy PET/CT in T/NK-cell lymphomas.
DISCLOSURE
The costs of publication of this article were defrayed in part by the payment of page charges. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734. This work was supported by the National Natural Scientific Research Fund of China (30400589 and 81071950), Fundamental Research Funds for the Central Universities (10ykpy36), Key Projects in the National Science and Technology Pillar Program during the Eleventh Five-Year Plan Period of China (2008ZX09312-002 and 2012ZX09301), National University Outstanding Young Teacher Support Program at Sun Yat-Sen University, and the Research Award Fund for Outstanding Young Researchers at Sun Yat-Sen University Cancer Center. No other potential conflict of interest relevant to this article was reported.
Acknowledgments
We thank the nuclear medicine physicians and radiologists of the Department of Nuclear Medicine at Sun Yat-Sen University Cancer Center.
Footnotes
↵* Contributed equally to this work.
Published online Feb. 8, 2013.
- © 2013 by the Society of Nuclear Medicine and Molecular Imaging, Inc.
REFERENCES
- Received for publication June 20, 2012.
- Accepted for publication September 27, 2012.