Abstract
In patients with carcinoma of the head and neck and of the esophagus, metabolic and functional imaging by PET with 18F-FDG has a pivotal role in the evaluation of tumor response to therapy, specifically, in the prediction of progression-free survival and overall survival. Metabolic imaging allows the detection of biochemical changes within tumor cells as opposed to identifiable morphologic changes. Anatomic imaging modalities do not reliably differentiate between responders and nonresponders early during the course of follow-up. The correlation between histopathologic tumor response after preoperative therapy and clinical prognosis is well established for many cancers. Squamous carcinoma of the head and neck and esophageal carcinoma demonstrate avid 18F-FDG uptake. For these cancers, 18F-FDG PET parallels histopathologic findings in its ability to detect residual viable tumor; therefore, it is a valuable tool for the noninvasive assessment of histopathologic tumor response in advanced-stage cases after neoadjuvant therapy before surgery. Early determination of nonresponders is of prime importance, as timely therapy modification can be accomplished for patients who do not demonstrate a response to therapy. This determination is exceptionally important for head and neck and esophageal malignancies, both of which are known for their unfavorable prognosis, as early modifications in therapy regimens for nonresponders may improve patient outcome. There is now evidence that 18F-FDG PET is a sensitive and specific method for determining therapy response and for providing important prognostic information for these cancers. Therefore, 18F-FDG PET may change patient management and lead to improved survival for a selected group of patients with carcinoma of the head and neck and of the esophagus.
The primary goal of cancer treatment is to produce a complete and sustained remission, which usually is judged based on changes in tumor volume after therapy accompanied by clinical relief of symptoms. Functional changes, such as tissue metabolism and physiologic functions, often predate structural changes in tissues; thus, the identification of residual viable tumor by anatomic imaging modalities usually is difficult, particularly when tumor cells are replaced by fibroblasts without significant loss in volume. The TNM staging system is also an inadequate indicator of individual responses to various cancer therapies. The extent of therapy-induced cellular injury in tumors can be determined with biologic markers as predictors of clinical outcome. However, approaches that use new cellular markers to predict the success of neoadjuvant therapy are not well established, and the literature is still inconsistent.
In carcinoma of the head and neck and of the esophagus, clinical response evaluation after treatment is restricted by the lack of noninvasive imaging modalities, which would allow for valid differentiation of responders from nonresponders. Individual patients display various sensitivities to treatments as a function of differences in tumor perfusion and molecular biology. Chemotherapy or combination therapy (chemoradiotherapy) is used to decrease tumor size before surgery in locally advanced-stage cases to render subsequent tumor resection feasible for organ-preserving surgery, in particular, for cases of carcinoma of the head and neck. These neoadjuvant approaches may result in improved tumor response and complete remission in some cases. Conversely, for other groups of patients, primary chemotherapy or radiotherapy (RT) may not demonstrate a clear benefit for local-regional tumor control and overall patient survival. Although evaluation of RT is somewhat different from that of chemotherapy because of the ionizing effects (1), observed differences in responses to these types of therapy between various groups of patients with advanced-stage disease have stimulated research to identify better predictors for therapy response.
There is an advantage to assessing therapy response early during chemotherapy, as early evidence of persistent disease in both head and neck cancer and esophageal cancer may provide a basis for innovative interventions for advanced stages. Early recognition of resistance to chemotherapy also can result in lower cumulative treatment toxicity and tumor burden. At present, PET with 18F-FDG can contribute to the detection of residual or recurrent tumors, leading to the early institution of salvage therapy or the prevention of unnecessary biopsies of irradiated tissues, which may aggravate injury. As a tumor metabolic marker, 18F-FDG PET can help clinicians choose optimal treatments for individual patients and maximize treatment efficacy in terms of cure and organ preservation.
In the present review, we have evaluated the role of 18F-FDG PET in predicting tumor response to therapy and its prognostic value for squamous cell carcinoma of the head and neck (HNSCC) and for esophageal carcinoma.
HEAD AND NECK CARCINOMA
Head and neck cancers represent a group that accounts for approximately 3%–5% of cancers in the adult population in the United States. The majority of head and neck malignancies are squamous cell carcinomas of the nasopharynx, oropharynx, oral cavity, and larynx. In patients with early-stage disease, both radiation and surgery often are curative, with similar cure rates. In patients with advanced HNSCC, however, the overall survival rate is about 40%, despite the evolution and refinement of combination treatments. Only 20% of patients with recurrent disease survive at 1 y, although this survival rate may change with effective therapy in selected patients (2).
Patient Population to Benefit from Evaluation of Response to Therapy
Previously Untreated Patients.
Early-stage (stages I and II) HNSCC can be cured by either surgery or RT. The “standard” treatment for locally advanced tumor (stages III and IV [M0]) has been surgery followed by RT (3,4). However, newer therapeutic approaches now are available; these include neoadjuvant (induction) chemotherapy followed by RT or neoadjuvant chemotherapy concurrent with RT and followed by surgery. The main advantage of neoadjuvant chemotherapy is the preservation of organ function, as downstaging by chemoradiotherapy allows for organ preservation. In a neoadjuvant setting, monitoring the response to therapy by 18F-FDG PET could lead to significant changes in patient management by accurately differentiating responders from nonresponders. In responders, the extent of surgery could be modified, and in nonresponders, surgical intervention could be avoided.
The rationale of postoperative adjuvant chemoradiotherapy is to eradicate residual primary tumor and possible microscopic metastatic sites. 18F-FDG PET does not offer much clinical benefit in this adjuvant therapy setting for monitoring the response to therapy. If a primary tumor is not completely resected at surgery, a postsurgical pretreatment 18F-FDG PET scan may produce false-positive findings because of the recent surgical intervention. In these circumstances, 18F-FDG PET is not reliable for accurately evaluating residual tumor and the subsequent therapy response. Furthermore, the resolution of currently available PET systems is not sufficiently high for the detection of microscopic residual disease.
Patients with Recurrent Disease.
The median survival time for patients with local or metastatic recurrent HNSCC is 6 mo (3). Combination chemotherapy regimens, second-course high-dose RT, and biologic therapy with cytokines, retinoids, antiangiogenic agents, and monoclonal antibodies have been developed in an attempt to improve survival in this patient population (5). 18F-FDG PET may have a role in the evaluation of the response to therapy for guiding various therapy strategies, although its impact may not be as significant in patients with recurrent disease as in those undergoing neoadjuvant therapy at initial presentation. However, if the success of the therapy could be predicted accurately by 18F-FDG PET, unnecessary morbidity from ineffective treatment could be avoided for patients who are predicted not to benefit. Alternatively, for patients showing a good response to therapy, no further consideration of management change would be necessary.
Evaluation of Responses to Chemotherapy and Combination Therapy
Markers of Response in Head and Neck Cancer.
Several cellular biomarkers provide information on treatment efficacy and survival rates in advanced HNSCC. These markers include vascular endothelial growth factor, cell proliferation index, and control of programmed cell death (bcl-2, p53, and bax expression) (6). Additionally, a correlation among glucose transporter protein GLUT-1, tumor hypoxia, and radioresistance has been reported (6). The role of these prognostic markers in defining the response to cytotoxic therapy, however, still remains poorly understood. Hence, a reliable response predictor for guiding therapy strategy and maximizing treatment efficacy has yet to be defined.
In the posttherapy setting, 18F-FDG PET provides confirmatory evidence of residual or recurrent disease. A multitude of studies have demonstrated a clear advantage of 18F-FDG PET imaging over anatomic imaging modalities in monitoring therapy response during or after therapy in HNSCC (7–19). The “gold standard” for identifying residual disease in HNSCC after therapy is fine-needle biopsy. Collins et al. reported a sensitivity of 94% for fine-needle aspiration biopsy and 18F-FDG PET in patients with suspected recurrence and local metastases of oropharyngeal carcinoma (14).
Chemotherapy and RT are associated equally with high morbidity rates; therefore, for individual treatment planning, it is important to evaluate accurately the effects of therapy during the follow-up period.
Early Prediction of Therapy Response.
Early identification of the response to therapy may lead to timely alterations in therapeutic strategy and salvage surgery in locally advanced HNSCC (Table 1).
18F-FDG PET Evaluation of Response to Therapy of Carcinoma of Head and Neck
Pretherapy 18F-FDG uptake measured as a standardized uptake value (SUV) may be a useful parameter to identify patients who require more aggressive treatment. Several studies have suggested that the degree of pretherapy 18F-FDG uptake can be used to stratify patients into high- and low-risk categories (15–18). A recent study by Allal et al. evaluated pretherapy 18F-FDG uptake as a predictor of local control and disease-free survival (DFS) after RT with or without chemotherapy in 63 HNSCC patients (18). Patients with high tumor 18F-FDG uptake (SUV, >5.5 mg/mL) had significantly lower 3-y local control (55% vs. 86%) and DFS (42% vs. 79%) rates than did patients with low uptake (SUV, ≤5.5 mg/mL). In a multivariate analysis, SUV was found to be an independent predictor of DFS, whereas tumor size at staging (T category) was of borderline significance. Although further confirmatory studies are warranted, pretreatment 18F-FDG PET findings may have prognostic implications in determining which patients will show long-term local control. Nevertheless, prediction of tumor response based on SUVs usually is impractical and challenging because of the large overlap between responders and nonresponders.
Evaluation of the response during therapy is useful as an early assessment of tumor response. There is, however, a paucity of 18F-FDG PET data obtained during the course of therapy in patients with HNSCC (Table 1). Several studies have shown a correlation between the decline in 18F-FDG uptake and tumor glucose metabolism during therapy and a similar sustained trend after therapy (7,12,16,19,20). A comparison of the various results is difficult, however, because of differences in methods of analyses (quantitation with SUVs, quantitation with kinetic models, and qualitative analyses) and the small numbers of patients studied in most of these investigations.
In a study by Reisser et al., an obvious therapy response was revealed by 18F-FDG PET after the first cycle of chemotherapy in 12 patients with advanced HNSCC (19). The investigators found a significant decrease in 18F-FDG uptake (>10%), a finding that is consistent with low early remission rates, in only 47% of the cases. The treatment responses varied in different lymph nodes in the same patient. This finding is suggestive of tumor heterogeneity, which is a well-known contributor to tumor treatment resistance. That study lent credence to other studies, but the shortcomings in the design of that study were the lack of long-term follow-up to determine whether complete responses were sustained and an inadequate number of patients to derive a firm conclusion.
It is widely known that a decrease in tumor dimension after therapy is not an accurate predictor of residual viable tumor. Several studies have indicated that the mean tumor volume reduction revealed by CT only approaches statistical significance (12,21). Compared with size changes seen on CT, a reduction in SUVs is statistically significant in determining therapy response (12). Dalsaso et al. prospectively evaluated 18F-FDG PET before and after 2 or 3 cycles of chemotherapy in 19 patients with stage III or IV HNSCC. Three patients who had a mean SUV reduction of 82% showed a complete pathologic response on biopsy. Not surprisingly, 16 patients who had an SUV reduction of only 32% were determined to have residual disease after chemotherapy (12).
In a larger series, 47 patients with stage II–IV HNSCC underwent 18F-FDG PET before and after 1 cycle of chemotherapy or after RT (median, 24 Gy) to predict therapy outcome, defined as tumor response, survival, and local-regional control (16). All patients received RT, and 10 patients also received neoadjuvant chemotherapy. After therapy, a high (≥16 μmol/min/100 g) metabolic rate for 18F-FDG was associated with complete remission in 62% of patients; remission occurred in 96% of patients with a low metabolic rate (<16 μmol/min/100 g). The 5-y overall survival rates in the groups of patients with low and high glucose metabolic rates were 72% and 35%, respectively. In that study, although SUV and metabolic rate for 18F-FDG produced similar results with respect to therapy response, a discrepancy between the 2 measures was found with increasing metabolic rate for 18F-FDG and SUV: SUV showed a poorer association with survival. This observation indicates that the metabolic rate for 18F-FDG may have a greater prognostic value than the SUV; however, given the small size of the patient group, low precision of the estimates also was possible.
Prediction of Therapy Response After Completion of Treatment.
It is always a challenge to determine the most optimal period for performing 18F-FDG PET imaging during the posttherapy follow-up period (10,12,13,19,21–23). It is of greater benefit for a patient to undergo 18F-FDG PET early during the course of follow-up in the interest of better patient management (Fig. 1). Immediately after therapy, however, 18F-FDG PET findings may be associated with high false-positive results because of the tissue healing process or false-negative results because of alterations in 18F-FDG uptake kinetics, particularly when RT is involved.
A 45-y-old man with invasive squamous cell carcinoma of left palatine tonsil. (A) Patient underwent 18F-FDG PET scan simultaneously with CT before initiation of therapy. Axial PET (middle) image reveals intense 18F-FDG uptake in left tonsil (vertical arrow) as well as in a left jugular lymph node (horizontal arrow), consistent with primary disease and local lymph node metastasis, respectively (locally advanced disease). Axial CT (left) and PET/CT fusion (right) images confirm these findings (arrows). Comprehensive examination was obtained by simultaneous CT and PET/CT, combining anatomic data with functional or metabolic information. (B) Same patient underwent 18F-FDG PET scan simultaneously with CT 1 mo after completion of neoadjuvant chemoradiotherapy. Axial PET (middle) and PET/CT (right) images demonstrate interval resolution of primary disease in left tonsil and metastatic disease in a left jugular lymph node, consistent with complete response to therapy. This patient subsequently underwent surgical resection and has been disease-free during follow-up period of 6 mo. Although further follow-up is necessary, 18F-FDG PET was valuable in determination of complete response to therapy. PET/CT studies were obtained on a GE Discovery LS unit—a PET/CT fusion system combining GE LightSpeed multislice CT and Advance NXi PET (GE Medical Systems).
After chemotherapy, persistent 18F-FDG uptake is a harbinger of therapy failure (Fig. 2). In one study by Lowe et al., 28 patients who had stage III or IV HNSCC and who participated in an organ preservation protocol underwent 18F-FDG PET before and after neoadjuvant chemotherapy (13). Tissue biopsy specimens were obtained for all patients before and after chemotherapy. The sensitivity and specificity of posttherapy 18F-FDG PET for detecting residual disease were 90% and 83%, respectively. Those who achieved complete remission had a mean reduction in 18F-FDG uptake of 82%; in comparison, those who had residual disease after therapy had a reduction of 34%. Two patients had negative biopsy findings and positive 18F-FDG PET studies, indicating persistent disease. Although tissue confirmation is considered the gold standard for the assessment of therapy response, biopsy-based methods are expensive, entail some patient risk, and may yield false-negative results because of sampling errors. 18F-FDG PET may avoid the risk of sampling errors; however, the accuracy of 18F-FDG PET data compared with that of biopsy data should be confirmed by larger studies.
A 50-y-old man with recurrent HNSCC in a cervical lymph node underwent PET/CT imaging before and after completion of chemotherapy. (A) Pretherapy axial CT (left) and PET (right) images reveal intense radiotracer uptake in a right jugular lymph node (arrow on PET image) in the same anatomic location as lymphadenopathy seen on corresponding CT image. (B) Posttherapy axial CT (left) and PET (right) images reveal persistent 18F-FDG uptake in the corresponding locations (arrow), consistent with residual disease and therapy failure. Patient’s disease subsequently further progressed. PET/CT studies were obtained on a GE Discovery LS unit—a PET/CT fusion system combining GE LightSpeed multislice CT and Advance NXi PET (GE Medical Systems).
Perie et al. prospectively evaluated the response to chemotherapy by using coincidence imaging (dual-head γ-camera) for 34 patients with HNSCC (22). For patients who underwent postchemotherapy surgical resection (n = 23), the rates of agreement with histopathologic results for response to treatment were 74%, 69%, and 78% for panendoscopy, CT, and 18F-FDG PET, respectively. For patients who underwent only biopsy before RT (n = 11), however, the rates of agreement with histopathologic results for response to treatment were 75%, 75%, and 67% for panendoscopy, CT, and 18F-FDG PET, respectively. After neoadjuvant chemotherapy, 18F-FDG PET was equivalent to CT in detecting metastatic cervical lymph nodes, with an accuracy of 93%. In that study, posttherapy evaluation of response by 18F-FDG PET was inferior to that by CT and panendoscopy for patients who underwent only biopsy. The reasons for this finding may be multifactorial and may include small sample size and tissue sampling errors. Furthermore, in that study, a dual-head coincidence camera instead of a dedicated full-ring PET system was used. Coincidence cameras have one third the sensitivity of dedicated PET systems (24). Given the superior sensitivity of dedicated PET scanners, these results may have been improved significantly with a dedicated PET system.
In an assessment of combination therapy with 20 patients who were monitored for a mean follow-up of 11 mo, Conessa et al. reported that the best time to perform 18F-FDG PET is 3–4 mo after therapy (25). In that study, 18F-FDG PET was performed at 3–6 mo after the completion of therapy that systematically included RT. Histologic confirmation was available for all patients. Among 8 negative 18F-FDG PET studies, only 1 patient developed cervical lymph node metastasis at 5 mo. In that series, 18F-FDG PET had a sensitivity of 87% and a specificity of 67% for detecting residual or recurrent disease. The specificity was relatively lower because of false-positive cases caused by posttherapy inflammatory processes.
In a recent study by Sakamato et al., 22 patients with HNSCC underwent 18F-FDG PET before and 3–4 wk after the completion of RT or chemotherapy (10). The posttherapy SUVs were significantly lower than the pretherapy values (mean, 7.0 vs. 3.8 mg/mL). Not surprisingly, the reduction in tumor size on concurrent CT or MRI was not relevant to patient outcome. The mean posttherapy SUVs in patients showing complete response (complete responders), partial response (partial responders), and no response were 2.7, 3.6, and 4.5 mg/mL, respectively. Based on the direct correlation between histopathologic findings and SUVs, it was recommended that a tumor be assumed to harbor viable cells if the posttherapy SUV exceeds 3 mg/mL. The findings of that study were confirmed by data obtained by Kitagawa et al. (17). In the latter study, it was reported that patients with posttreatment tumor SUVs of >4 mg/mL would be more likely to have persistent disease than would those with SUVs of <4 mg/mL. There was an overlap, however, for SUVs of about 3 mg/mL between some patients with and those without viable tumor cells. This overlap may have been attributable to the relatively early posttherapy period of assessment, when tissues are still in the process of recovering from RT-induced inflammatory changes. Therefore, false-positive findings are more likely to occur during this early period than during later periods of assessment (10).
Goerres et al. systematically reviewed the accuracy of 18F-FDG PET in the follow-up of patients with head and neck cancer and found that the main advantage of 18F-FDG PET is the ability to reliably rule out the presence of disease at restaging (26). In the same context, the impact of 18F-FDG PET on patient management was evaluated in another study by use of physician surveys for a conglomerate group of patients with head and neck, lung, and colorectal carcinomas (27). 18F-FDG PET positively affected surgery in 58% of patients, prompted the addition of chemotherapy or RT in 17%, and eliminated chemotherapy or RT in 8%. Overall, 18F-FDG PET affected patient management in 70% of the cases and had some decision-making value in another 26%. Hence, the sensitivity and specificity of 18F-FDG PET metabolic imaging, when combined with complementary anatomic imaging techniques, contribute significantly to the clinical management of cancer patients, including those with HNSCC. Nonetheless, further confirmatory research is needed to link the influence of 18F-FDG PET on patient management to cost-effectiveness.
Evaluation of Response to RT
Monitoring RT during the course of therapy is more complex than monitoring chemotherapy because of differences in the cell-killing mechanisms. Ionizing radiation absorbed by human tissues has enough energy to remove electrons from the atoms that make up molecules of the tissue. A molecule disintegrates when the electron that is shared by the 2 atoms to form a molecular bond is dislodged by ionizing radiation. The time at which injury occurs is related to the rate of normal cell proliferation (1). In rapidly proliferating tissues, such as epithelial surfaces, injury usually is noted within 3 wk after irradiation. If sufficient cells survive irradiation, injury will be repaired by cell proliferation and effective cell repair mechanisms. A reliable technique for use as a prognostic indicator of response to RT has yet to be determined; such a technique may make future treatments more predictable.
When normal mammalian cells are subjected to stress signals, such as radiation, chemotherapeutic drugs, and oxygen deficiency, a range of gene products involved in the sensing and signaling of such stresses are activated. The response of human cells to ionizing radiation includes the activation of DNA repair pathways and cell cycle checkpoints, with subsequent full biologic recovery or cell death. Radiation induces 2 modes of cell death, termed mitotic or clonogenic cell death and apoptosis. There have been recent major advances in the understanding of the signal transduction pathways involved in determining the fate of cells after irradiation. Damage to DNA constitutes the basis of cell lethality that eventually results in apoptosis or necrosis.
The timing of posttreatment 18F-FDG PET is of prime importance for accurate response evaluation (8,28). Rege et al. endeavored to determine the value of 18F-FDG PET in the prediction of local control and overall survival for 12 patients who had HNSCC and were undergoing primary RT (28). All patients underwent 18F-FDG PET scans before, during, and 6 wk after the completion of RT. Tumors with a >50% decrease in metabolic activity after RT showed improved local control. 18F-FDG uptake in the primary tumor increased early during the course of RT (<20 Gy) but decreased near the end of therapy (>45 Gy), whereas there was no significant change in normal structures of the neck after 6 wk of RT. Tumor 18F-FDG uptake decreased in all responding cases, but in therapy-refractory tumors, glucose metabolism increased after 6 wk of treatment. The authors concluded that persistent uptake on 18F-FDG PET images obtained 1 mo after RT strongly suggests residual tumor and that tissue changes immediately after the initiation of RT may lead to false-positive findings (28).
There is also a potential risk that RT may cause false-negative findings by changing 18F-FDG uptake kinetics. Greven et al. performed 18F-FDG PET before, 1 mo after (n = 22), and 4 mo after (n = 18) RT for patients with HNSCC (8). At 1 mo after therapy, histopathologic findings revealed residual tumor in 6 patients, all of whom had positive 18F-FDG PET studies. There were, however, 3 false-negative 18F-FDG PET studies during this evaluation period. At 4 mo after therapy, 18F-FDG PET studies were true-positive for all patients with residual tumor revealed by histopathologic findings. No patient with a negative 18F-FDG PET study relapsed during this evaluation period. Thus, it appears that early evaluation—at 1 mo after the completion of RT—may produce false-negative results. It is possible that immediately after RT, the incorporation of 18F-FDG into tumor cells is decreased regardless of tumor viability. It was postulated that the radiation-induced changes in 18F-FDG uptake might have been attributable to altered cellular glucose transport mechanisms related to GLUT-1, hexokinase, or vascular damage rather than cell death (29). It is possible that an 18F-FDG PET study carried out after 1 mo but before 4 mo may differentiate responders from nonresponders when other clinical indicators demonstrate equivocal results.
In a more recent study, Kunkel et al. reported that post-RT 18F-FDG uptake predicts survival and local tumor control (30). The authors analyzed the prognostic significance of glucose metabolism after neoadjuvant RT (36 Gy) immediately before tumor resection. The 3-y survival rates were 80% in the group with a low SUV (<4 mg/mL) and 43% in the group with a high SUV (≥4 mg/mL). A high 18F-FDG uptake was associated with an increased death rate and local progress even when radical resection was performed. Thus, 18F-FDG PET accurately identifies patients who have a poor prognosis and for whom radical surgery must be considered with caution.
A pilot study carried out by Slevin et al. suggested the superiority of 18F-FDG PET over MRI for 21 patients in the post-RT assessment of tumor response (31). Patients had pretreatment 18F-FDG PET and MRI scans, and these were repeated after 4 and 8 mo of RT. A discordance of posttreatment 18F-FDG PET and MRI findings in 1 case indicated a possible role for 18F-FDG PET in the early detection of tumor recurrence. Nonetheless, the number of patients should be expanded to extrapolate statistically meaningful conclusions from these preliminary studies.
False-Positive Results
Radiation and chemotherapy induce diffusely elevated 18F-FDG accumulation within normal tissues because of inflammatory changes. Therapy causes an inflammatory or leukocytic infiltrate that consists of neutrophils, lymphocytes, and macrophages as well as proliferating fibroblasts. After RT, these posttherapy changes are most prominent in the epithelial surfaces of the oral mucosa, soft palate, paranasal sinuses, and palatine tonsils. Additionally, reactive lymph nodes, tense cervical muscles, and active laryngeal muscles (attributable to vocalization) may cause false-positive results. Asymmetric laryngeal muscle activity mimicking a metastatic cervical lymph node may occur because of unilateral recurrent laryngeal nerve paralysis (32). Hence, patients should be instructed not to talk starting 15 min before and until 15 min after the injection, so that the muscles involved in speech do not accumulate 18F-FDG.
False-Negative Results
18F-FDG PET studies performed earlier than 4 mo after the completion of RT may cause false-negative results (8). Additionally, 18F-FDG PET imaging can underestimate metabolic activity in tumors that are smaller than 2 times the spatial resolution of the scanner. Thus, tumors smaller than 0.5 cm may not be detected by currently available PET scanners.
Other PET Radiotracers and Special Considerations for HNSCC
Tumor Hypoxia.
Hypoxic cells are more resistant to RT than are more highly oxygenated cells and therefore require up to 3 times as much radiation to experience an equivalent level of cytotoxicity. Consequently, local-regional tumor control of locally advanced HNSCC with RT has been unsatisfactory in part because of the phenomenon of tumor hypoxia. Hence, it would be useful to evaluate hypoxia before therapy to predict treatment efficacy and to overcome inherent hypoxia-induced radioresistance. However, assessing hypoxia in human tumors has proven difficult because of the lack of noninvasive and reproducible methods. As a noninvasive hypoxia imaging method, PET has been investigated with both imidazole- and non-imidazole-based agents (33,34). The most extensively used radiotracer is 18F-fluoromisonidazole (18F-FMISO), which acts as a bioreductive molecule and is incorporated into cell constituents under hypoxic conditions. However, because of unfavorable pharmacokinetics, such as slow cellular uptake and slow washout from nonhypoxic tissues, 18F-FMISO has not received wide clinical acceptance as a means for measuring tumor oxygenation. In a recent study, 18F-FMISO was used to monitor tumor hypoxia after concomitant chemotherapy and RT in 15 patients with HNSCC (33). The investigators showed normalization of 18F-FMISO activity after therapy in responding patients. However, there are no data analyzing the relationship between tumor 18F-FMISO uptake before therapy and ultimate clinical outcome. The predictive value of baseline 18F-FMISO uptake in human tumors is unknown (33,34).
A recently developed PET-based hypoxia measurement technique that uses a 62Cu(II)-diacetyl-bis[N(4)-methylthiosemicarbazone] (62Cu-ATSM) tracer has evoked great interest. 62Cu-ATSM selectively accumulates in hypoxic tissues, where it is reduced, whereas it is cleared rapidly from nonhypoxic tissues. Chao et al. examined the feasibility of 62Cu-ATSM-guided intensity-modulated RT, which may deliver a higher dose of radiation to the hypoxic tumor volume (35). The investigators demonstrated the feasibility of 62Cu-ATSM-guided intensity-modulated RT by showing that the dose of radiation to the hypoxic gross tumor volume could be escalated without compromising normal tissue protection in HNSCC. The plan delivered 80 Gy in 35 fractions to the 62Cu-ATSM-avid tumor subvolume, and the gross tumor volume simultaneously received 70 Gy in 35 fractions; more than one half of the parotid gland tissue was spared. Nonetheless, a pathologic correlation between 62Cu-ATSM retention and radiation curability is necessary for understanding tumor reoxygenation kinetics during a course of RT before this therapeutic approach can be implemented for locally advanced HNSCC.
Amino Acid Metabolism.
11C-Methionine (11C-MET) also has been evaluated as a marker for amino acid metabolism for monitoring the effects of therapy by PET. The exact mechanism of uptake is unknown, but factors influencing uptake probably include membrane amino acid transport, integrity of the blood-brain barrier, and protein synthesis. Nuutinen et al. reported that 11C-MET uptake significantly decreased during the first 2–3 wk of RT in HNSCC (36). When a threshold SUV of 3.1 mg/mL was used, complete responders could be separated from nonresponders. Unfortunately, it appears that the rates of decrease in 11C-MET uptake are similar between relapsing tumors and those that remain in remission. Thus, the use of 11C-MET to predict a response to RT is somewhat limited and warrants further investigation.
Angiogenesis.
Angiogenesis is a target for the treatment of cancer, and its complex biology suggests that establishing the appropriate dose and schedule for antiangiogenic treatment will require extensive studies. A phase 1 dose-escalating clinical trial of recombinant human endostatin (rh-Endo) investigated potential surrogates for a response to antiangiogenic therapy. Twenty-five patients were treated with escalating doses of rh-Endo. PET was used to assess tumor blood flow (with 15O-H2O) and metabolism (with 18F-FDG) before the start of therapy and then every 4 wk thereafter (37). Biopsy confirmation was available at 8 wk to evaluate for endothelial cell or tumor cell apoptosis. Tumor blood flow and glucose metabolism generally decreased with increasing doses of rh-Endo; however, the effects were not straightforward. There was no statistically significant relationship between rh-Endo dose and induction of tumor cell or endothelial cell apoptosis. These initial data, however, suggest that rh-Endo has measurable effects on tumor blood flow and metabolism and induces endothelial cell or tumor cell apoptosis even in the absence of demonstrable anticancer effects. Further study and validation of such biomarkers are required for a better understanding of antiangiogenic therapy.
Conclusion
18F-FDG PET is a valuable monitoring tool for patients undergoing preoperative induction chemotherapy and RT for locally advanced HNSCC. 18F-FDG PET has been reported to provide crucial information by accurately differentiating responders from nonresponders before surgery in various series. Hence, 18F-FDG PET may change clinical management after neoadjuvant therapy significantly by optimizing surgical treatment for each patient. Additionally, therapy-associated morbidity can be avoided in patients for whom therapy will fail. Furthermore, patients at increased risk of recurrence may benefit from more aggressive therapy schemes or combined-therapy options early during the course of disease. Nevertheless, longer follow-up and large series of patients should be evaluated to establish firmly the prognostic value of 18F-FDG PET for HNSCC.
ESOPHAGEAL CARCINOMA
Approximately 50% of esophageal cancers are squamous cell carcinomas. Adenocarcinomas, typically arising in Barrett’s syndrome of the esophagus, account for the other 50% of malignant lesions, and the worldwide incidence of this histologic finding appears to be rising. The 5-y survival rates after surgical resection are only 10%–35% (38). Despite a significant decrease in surgical mortality rates in patients with esophageal cancer, long-term survival rates have not changed dramatically. Recent evidence reveals that the addition of neoadjuvant treatment may improve resection rates, reduce recurrence risk, and thereby improve survival. Because of the poor prognosis and the risks associated with surgical intervention, an accurate assessment of therapy is essential for optimal treatment planning.
Patient Population to Benefit from Evaluation of Response to Therapy
Previously Untreated Patients.
The benefit of a combination of neoadjuvant chemotherapy and RT before surgical resection has been associated with improved DFS and overall survival for patients who have a tumor-free surgical specimen. The rationale behind such treatment is to improve the rate of curative resection by tumor downstaging, early eradication of micrometastases, and an increase in radiosensitivity for patients with locally advanced esophageal cancer (T3 or T4 status; stage III). Improved 5-y survival rates approaching 60% have been reported after complete pathologic response with concomitant RT and chemotherapy (39). 18F-FDG PET is particularly useful for patients undergoing neoadjuvant therapy, as the use of this technique allows accurate stratification of patients into surgical and combined-therapy protocols. This aspect is important, because approximately 50% of patients do not respond to currently available chemotherapy regimens (40). For patients who will not show a response to neoadjuvant treatment, the risk of treatment-related morbidity and mortality can be avoided if 18F-FDG PET can accurately determine therapy failure. Furthermore, these patients can be placed on alternative therapies early during the course of disease.
At present, the role of postoperative adjuvant chemoradiotherapy is undefined, although it may be beneficial in patients with positive resection margins. As with HNSCC, in an adjuvant therapy setting, the role of 18F-FDG PET is limited to monitoring therapy response.
Patients with Recurrent Disease.
Two thirds of patients have a recurrence within 1 y, and in approximately one third of patients, the recurrence occurs within the primary surgical field (Fig. 3) (41). 18F-FDG PET may provide a highly sensitive and specific means for distinguishing postoperative scar from tumor recurrence in the follow-up period. 18F-FDG PET also is valuable for further investigation of abnormal masses seen by other imaging modalities, especially in asymptomatic patients. Treatment of recurrent esophageal cancer may include palliative therapy with any standard treatment as well as clinical trials of novel therapies. Although evaluation of the response to therapy with 18F-FDG PET is of limited value, it may allow early changes in the treatment of unresponsive tumors or a discontinuation of therapy in nonresponding patients.
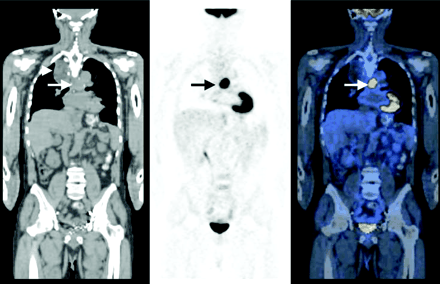
A 62-y-old man with history of locally advanced adenocarcinoma of distal esophagus after neoadjuvant therapy, with partial response, determined by outside 18F-FDG PET study. Patient had undergone esophagectomy and gastric pull-up surgery and now was referred for 18F-FDG PET scan to evaluate disease status 6 mo after completion of neoadjuvant therapy and surgery. Coronal CT (left), PET (middle), and PET/CT (right) images demonstrate intense 18F-FDG uptake in midline in midchest, corresponding to tracheobronchial lymph nodes, consistent with metastatic disease (arrows). Patient’s disease progressed, and patient died within 3 mo after study. This study emphasizes that 18F-FDG PET after neoadjuvant therapy appears to predict prognosis with high accuracy; median survival time of nonresponders is much shorter than that of responders. Note postsurgical anatomic changes in right upper chest secondary to gastric pull-up surgery on CT image (left; arrowhead). PET/CT studies were obtained on a GE Discovery LS unit—a PET/CT fusion system combining GE LightSpeed multislice CT and Advance NXi PET (GE Medical Systems).
Evaluation of Responses to Chemotherapy and Combination Therapy
Markers of Response in Esophageal Cancer.
Preoperative chemotherapy in patients with esophageal cancer is hampered by the lack of reliable predictors of tumor response. For squamous cell carcinoma, the cellular response markers that have been investigated are proliferating cell nuclear antigen and epidermal growth factor receptors, p53 protein expression, and Ki-67 antigen expression in biopsy specimens (42,43). For adenocarcinoma, c-erb B-2 protein expression and posttreatment transforming growth factor α expression have been reported to predict therapy response and overall survival (44,45). Nevertheless, the role of these molecular markers is not well established, and the data are in evolution. Noninvasive markers of tumor response would evoke great clinical interest.
After therapy, the esophageal wall often appears abnormal regardless of the degree of therapy response. Therefore, distinguishing residual tumor from therapy-related changes on anatomic imaging modalities, including CT, MRI, endoscopic ultrasound, and endoscopic MRI, usually is not possible (39). In this scenario, 18F-FDG PET offers a unique opportunity to evaluate the effectiveness of preoperative therapy by visualizing changes in tumor metabolic activity (Fig. 3).
Early Prediction of Therapy Response.
Neoadjuvant therapy has the potential to improve survival; however, response rates have not reached expectations. Early evaluation of therapy response may improve therapy efficacy through accurate differentiation of responders from nonresponders. The results of several studies have indicated that changes in SUVs observed during treatment correlate strongly with therapy response and also predict the risk of local recurrence and overall survival (Table 2) (46,47). In a preliminary study by Couper et al., 14 patients who had esophageal or gastric cancer and who received combination therapy underwent 18F-FDG PET before and after 2 or 3 cycles of chemotherapy (46). The 18F-FDG tumor-to-liver uptake ratios ranged from complete resolution to a 15% increase. In patients with increased 18F-FDG uptake after therapy, there was agreement for disease progression among 18F-FDG PET, CT, and clinical assessment. None of the patients with a reduction of <30% in uptake ratios had CT evidence of a response, although some of them had improvements in dysphagia scores. In the 6 patients with a reduction of >30% in uptake ratios, CT showed evidence of a response in only 67% (n = 4). Early clinical follow-up data confirmed the findings of 18F-FDG PET imaging. The shortcomings in the design of that study were the small number of patients and the lack of long-term follow-up data for most patients. The shortest survival was observed for a patient who had increased posttherapy tumor 18F-FDG uptake. Two patients with a reduction in 18F-FDG uptake of >30% were disease free for more than 15 mo. Hence, the authors concluded that 18F-FDG PET may play a major role in the assessment of a response to neoadjuvant therapy by allowing better selection of patients for continuation of treatment (46).
18F-FDG PET Evaluation of Response to Therapy of Carcinoma of Esophagus
Weber et al. reported that metabolic measurements with 18F-FDG PET allow early differentiation of responders from nonresponders during preoperative chemotherapy (47). The authors prospectively monitored 37 consecutive patients with locally advanced adenocarcinoma of the esophagus during the course of chemoradiotherapy. The patients underwent 18F-FDG PET studies at baseline and 14 d after the initiation of cisplatin-based chemotherapy. Clinical response, as evidenced by reduction of tumor length and wall thickness by >50%, was evaluated after 3 mo of therapy with endoscopy and anatomic imaging modalities. The reduction of tumor 18F-FDG uptake after 14 d of therapy for responding tumors was significantly different from that for nonresponding tumors (mean, 54% vs. 15%). When a cutoff value of 35% was applied as a criterion for a metabolic response, 18F-FDG PET predicted therapy response with a sensitivity and a specificity of 93% and 95%, respectively. The mean survival of responders was not reached during the 2-y period, whereas the mean survival for nonresponders was 13 mo (47).
Prediction of Therapy Response After Completion of Treatment.
Multiple studies have reported that 18F-FDG PET is a valuable tool for the noninvasive assessment of a histopathologic tumor response after the completion of neoadjuvant therapy in locally advanced esophageal cancer (Table 2) (48–52).
The cumulative data indicate that the response assessed by serial 18F-FDG PET scans after chemoradiotherapy correlates strongly with the pathologic response and survival (48–50). In a study by Flamen et al., 36 patients with locally advanced esophageal cancer (clinical T4 stage) but without organ metastases underwent 18F-FDG PET before and 1 mo after chemoradiotherapy (48). Patients were classified as responders when posttherapy 18F-FDG PET demonstrated a reduction in tumor-to-liver uptake ratios of >80%. The sensitivity and specificity of serial 18F-FDG PET studies for therapy response were 71% and 82%, respectively. After chemoradiotherapy, the median survival times of responders and nonresponders were 16.3 and 6.4 mo, respectively. A strong correlation was found between the extent of lymph node involvement shown by pretherapy 18F-FDG PET and the rate of a major response. The metabolic response measured by changes in posttherapy 18F-FDG uptake ratios was found to be a stronger prognostic factor for overall survival than was the extent of lymph node involvement determined by pretherapy 18F-FDG PET.
Changes in SUVs after induction therapy could accurately determine the response to therapy (49,50). In a study by Brucher et al., histopathologic evaluation revealed fewer than 10% viable tumor cells in responders when 18F-FDG PET was performed 3 wk after the completion of neoadjuvant chemoradiotherapy for squamous cell carcinoma of the esophagus (49). In responders, 18F-FDG uptake decreased by 72% ± 11% (mean ± SD), whereas in nonresponders, the decrease was only 42% ± 22%. At a threshold of a 52% decrease in the SUV, the sensitivity, specificity, and positive and negative predictive values for the prediction of therapy response were 100%, 55%, 72%, and 100%, respectively. Patients determined by 18F-FDG PET criteria to be nonresponders had significantly worse survival after resection than did responders. Kato et al. retrospectively assessed the performance of 18F-FDG PET compared with CT, endoscopy, and esophagography for monitoring therapy in advanced esophageal squamous cell carcinoma (50). Assessment of the rate of decrease in the SUV (>50% decrease) revealed a partial response in 50% of the patients, and assessment of the duration of the decrease in 18F-FDG uptake showed a partial response in 90% of the patients. Significant associations were observed between histopathologic response and tumor length, SUV after neoadjuvant therapy, and decrease in 18F-FDG uptake. However, the histopathologic response did not correlate significantly with the rate of decrease in the SUV for both CT and esophagography.
In further support of these previous data, Downey et al. recently reported that changes between pre- and posttherapy SUVs were predictive of DFS and overall survival in patients who had untreated carcinoma of the distal esophagus and who were eligible for induction therapy followed by resection (52). All patients undergoing esophagectomy after induction therapy were monitored for a disease-free interval of at least 24 mo or to recurrence. The median decrease in the SUV during induction therapy was 59%. After esophagectomy, the 2-y DFS and overall survival rates were 38% and 63%, respectively, in patients who had a decrease in the SUV of <60%. The respective values in patients who had a decrease in the SUV of >60% were 67% and 89%. After the completion of induction therapy, 18F-FDG PET did not detect new sites of metastatic disease and did not define nonresectable local-regional disease. Thus, the potential benefit of 18F-FDG PET studies performed after induction therapy was merely the assessment of the effectiveness of initial therapy. Patients with small decreases in 18F-FDG uptake after induction therapy were more likely to have disease recurrence than were patients with larger changes in 18F-FDG uptake. Hence, these findings suggest that changes in 18F-FDG uptake may predict DFS after neoadjuvant therapy. Further evaluations in larger trials are necessary to assess the role of 18F-FDG PET in predicting survival after induction therapy.
Evaluation of Response to RT
After neoadjuvant RT, the decline in 18F-FDG uptake can characterize tumor response; however, differentiating partial responders from complete responders can be difficult during or immediately after RT because of inflammatory changes, as described above. Nakamura et al. endeavored to differentiate nonresponders from complete responders by using 18F-FDG PET for 12 patients with squamous cell carcinoma of the esophagus (51). The patients underwent 18F-FDG PET studies before and immediately after RT. There was a significant difference in the median SUVs between nonresponders and complete responders (4.1 vs. 2.7 mg/mL). Three of the responders showed local-regional recurrence after 4, 6, and 18 mo. In the responders, the SUVs were not significantly different between patients with local recurrence and the remainder of the patients (51). These results obtained with squamous cell carcinoma of the esophagus are in partial agreement with the previously reported concept that the therapy response may not be a good predictor of survival for squamous cell carcinoma (53). After therapy, there is a greater chance for a complete response in patients with squamous cell carcinoma than in those with adenocarcinoma; however, the outcome for complete responders with adenocarcinoma appears to be better (60%) than that for complete responders with squamous cell carcinoma (40%). Nevertheless, patients with residual disease had a 5-y survival rate of 10%. These preliminary results warrant validation in larger trials and, if confirmed, several novel treatment strategies may be considered, including the use 18F-FDG PET to evaluate the effectiveness of induction therapy after only 1 cycle of induction therapy so that treatment can be altered or discontinued if necessary (52).
False-Positive Results
Gastric mucosa may demonstrate significant 18F-FDG uptake, posing potential confusion for tumors of the gastroesophageal junction. Various grades of 18F-FDG uptake may be seen in the normal esophagus, possibly because of smooth muscle activity or reflux esophagitis. In patients who have undergone recent RT, 8–12 wk should elapse before an 18F-FDG PET study is done to avoid false-positive findings of radiation-induced esophagitis. After esophagectomy, a photopenic defect to the right of the mediastinum is seen as a result of the gastric pull-up (stomach is pulled up into the chest) procedure. Surgery performed 4 wk before scanning may result in false-positive 18F-FDG uptake in areas of active inflammation. It is best to evaluate postsurgical patients at least 6 wk after surgery (54).
False-Negative Results
When disease is located near sites of physiologic uptake (heart, bladder, kidneys, and liver), 18F-FDG PET should be complemented by other imaging modalities to minimize false-negative findings. Because of the limited spatial resolution of PET, lesions smaller than 0.5 cm may be undetectable, particularly in the local-regional lymph nodes, where sensitivity has been reported to be only 45% because of overwhelming uptake at the primary site (55).
Other PET Radiotracers for Esophageal Cancer
Recently, a new PET tracer, 11C-choline (11C-CCH), was found to be useful for tumor detection. Many cancers, including esophageal cancer, are characterized by increased choline influx into the cells to meet the need for the increased synthesis of phosphatidylcholine, which constitutes the main component of cell membranes. Because tumor cells duplicate faster than do normal cells, the level of uptake of 11C-CCH in a tumor represents the rate of tumor cell duplication. Once phosphorylated, the polar phosphocholine molecule is trapped within the cells, providing a potential mechanism for the enhanced accumulation of radiolabeled choline. Although 11C-CCH has not yet been used as a tool for monitoring therapy, promising results were reported for the staging of esophageal cancer (54,56). In a recent study, 18F-FDG PET was able to detect 100% of malignant primary esophageal lesions, whereas 11C-CCH PET detected 73%. On the basis of these data, 11C-CCH PET appears to be able to visualize esophageal carcinoma and its metastases but appears to lack the sensitivity of 18F-FDG PET. These characteristics presumably are the result of low tumor uptake and considerable nonspecific background accumulation of 11C-CCH in the liver, stomach wall, pancreas, and small intestine (56).
Conclusion
Although studies performed so far lack long-term follow-up data and large patient populations, 18F-FDG PET imaging appears to be reliable in the evaluation of therapy response in patients who have locally advanced esophageal carcinoma and who are undergoing neoadjuvant therapy before surgery. The decrease in 18F-FDG uptake has been found to be significantly greater in patients who respond to therapy than in those who do not respond to therapy. This important information provided by 18F-FDG PET may enable the accurate differentiation of responders from nonresponders, thereby allowing better selection of patients for continued therapy. However, the difference in prognosis between partial responders and complete responders determined by PET criteria should be investigated in future studies. As sufficiently long-term follow-up has become available, it appears that 18F-FDG PET may be able to estimate the most important endpoints, that is, DFS and overall survival.
PET/CT FUSION IMAGING AND MONITORING OF THERAPY RESPONSE
PET/CT fusion imaging, which integrates 2 disparate imaging modalities, is now gaining momentum toward becoming a diagnostic tool for the staging and restaging of cancer. The primary advantage of PET/CT fusion technology is the ability to correlate 2 contemporaneous imaging modalities for a comprehensive examination that combines anatomic data with functional or metabolic information. After therapy, subtle metabolic findings on 18F-FDG PET that otherwise would have been disregarded may result in the detection of residual disease after correlation with simultaneously acquired morphologic data. Alternatively, equivocal CT findings, which could represent either recurrent tumor or posttherapy fibrosclerosis, now can be distinguished with the help of the additional information provided by 18F-FDG PET data. In addition to patient convenience, the CT component of fusion imaging can be used for attenuation correction of the PET study, a factor that considerably shortens the overall examination time (by ∼20 min).
18F-FDG PET has a well-established role in the restaging of cancer as well as in monitoring the response to therapy, and PET/CT may extend that role. In the posttherapy setting, PET/CT can improve the accuracy of PET imaging in distinguishing recurrent disease from benign posttherapy changes, delineating the anatomic location of metastatic disease, and monitoring therapy response by solving a myriad of problems inherent in the posttherapy assessment of cancer.
As PET/CT systems are not yet in widespread use, data regarding the impact of fusion imaging on posttherapy patient management are still in evolution. Nevertheless, preliminary data indicate that PET/CT findings have resulted in accurate staging and improved evaluation of the response to therapy, in particular, in patients with head and neck cancer and genitourinary and gastrointestinal malignancies (57,58).
In a recent study, the accurate spatial localization offered by PET/CT provided a better assessment of the response to treatment and changed clinical management in up to 30% of cancer patients (57). PET/CT provides accurate information about anatomic planes and excludes false-positive findings. There are fewer anatomic landmarks in the neck than in other parts of the body. Therefore, combining PET with CT provides crucial information in differentiating physiologic activity from viable tumor in the cervical muscles, brown fat at the base of the neck (58), vocal cords, lymphoid tissues, mucosal surfaces, and salivary glands, as all of these locations can demonstrate nonspecific high 18F-FDG uptake. In patients who have paralysis of the recurrent laryngeal nerve, unilaterally increased 18F-FDG uptake in the posterior arytenoid muscle in the nonparalyzed vocal cord also can be a potential source of false-positive findings without the guidance of PET/CT (37).
In conclusion, PET/CT has been reported to increase diagnostic confidence compared with either PET or CT imaging alone. The role of 18F-FDG PET may be significant in differentiating between benign tissue changes and persistent residual tumor; however, further studies are necessary to establish its role in the evaluation of the response to therapy.
SUMMARY
The planning of new and more intensive treatments is justified for chemosensitive or radiosensitive patients, but the question remains as to whether this subgroup can be predetermined before the introduction of unnecessary morbidity associated with therapy. The development of alternative treatment regimens in clinical oncology has increased the need for early prediction of cancer therapy outcome. In advanced HNSCC and esophageal cancer, neoadjuvant therapy has the potential of improving patient survival despite the reported low response rates in published series. Early detection of tumor response either during or at completion of therapy may improve the value of the neoadjuvant approach by determining which patients can benefit from therapy. More importantly, detecting patients for whom therapy will fail means that therapy can be avoided before the introduction of significant morbidity. Convincing evidence that 18F-FDG PET can predict ultimate prognosis in patients with HNSCC or esophageal cancer has been presented. Nonetheless, data from prospective randomized studies are needed to define better the role of 18F-FDG PET in differentiating responders from nonresponders.
Footnotes
Received Aug. 8, 2003; revision accepted Oct. 14, 2003.
For correspondence or reprints contact: Lale Kostakoglu, MD, New York Presbyterian Hospital, Weill Cornell Medical Center, 525 E. 68th St., Starr no. 221, New York, NY 10021.
E-mail: lak2005{at}med.cornell.edu
*NOTE: FOR CE CREDIT, YOU CAN ACCESS THIS ACTIVITY THROUGH THE SNM WEB SITE (http://www.snm.org/education/ce_online.html) UNTIL JANUARY 2005.