Abstract
This prospective study aimed to compare the diagnostic performance of 18F-fluorocholine and 18F-FDG for detecting and staging hepatocellular carcinoma (HCC) in patients with chronic liver disease and suspected liver nodules. Methods: Whole-body PET/CT was performed in a random order at 10 min after injection of 4 MBq of 18F-fluorocholine per kilogram and at 1 h after injection of 5 MBq of 18F-FDG per kilogram. PET/CT results were read in a masked manner by 2 specialists, and diagnostic performance was assessed from the results of consensus masked reading. Those focal lesions appearing with increased or decreased activity, compared with background, on 18F-fluorocholine PET/CT were considered positive for malignancy. The standard of truth was determined on a per-site basis using data from a histologic examination and a follow-up period of more than 6 mo; on a per-patient basis, the Barcelona criteria were also accepted as a proof of HCC in 5 patients. Results: Eighty-one patients were recruited; standard of truth was determined in 59 cases. HCC was diagnosed in 34 patients. Therefore, sensitivity was 88% for 18F-fluorocholine and 68% for 18F-FDG (P = 0.07), and in 70 sites, sensitivity was 84% for 18F-fluorocholine, significantly better than the 67% for 18F-FDG (P = 0.01). Of the 11 patients with well-differentiated HCC, 6 had a positive result with 18F-fluorocholine alone, whereas 18F-FDG was never positive alone; corresponding site-based sensitivity was 94% for 18F-fluorocholine and 59% for 18F-FDG (P = 0.001). The detection rate of 18 sites corresponding to other malignancies was 78% for 18F-fluorocholine and 89% for 18F-FDG. In nonmalignant sites, 18F-fluorocholine appeared less specific than 18F-FDG (62% vs. 91% P < 0.01) because of uptake by focal nodular hyperplasia. Conclusion: 18F-fluorocholine was significantly more sensitive than 18F-FDG at detecting HCC, in particular in well-differentiated forms. In contrast, 18F-FDG appeared somewhat more sensitive at detecting other malignancies and was negative in focal nodular hyperplasia. Thus 18F-fluorocholine appears to be a useful PET/CT tracer for the detection and surveillance of HCC; however, performing PET/CT with both radiopharmaceuticals seems to be the best option.
Hepatocellular carcinoma (HCC) is the fifth most common neoplasm in the world and the leading cause of death among cirrhotic patients. Any focal liver lesion in a patient with cirrhosis is suggestive of HCC, and early detection may permit curative treatment in 30%–40% of patients (1). α-fetoprotein (AFP) assay is the most frequent biologic screening test, but the diagnostic performance is poor. The radiologic modality most widely used for screening is ultrasonography, with a sensitivity around 60% but definitely lower for small nodules (2). A better sensitivity is obtained with contrast-enhanced CT, around 70%, and MRI, around 80% (3). However, an additional 30%–50% of unknown intrahepatic sites of HCC (mostly < 2 cm) are found at transplantation (4). The coupling of CT with PET brings a complementary metabolic approach to the characterization of nodules that can be useful, in particular in small nodules between 0.7 and 2 cm.
MRI and CT are currently also used during posttreatment monitoring of hepatic tumors, for residual disease and recurrence. PET has been proposed as a better imaging tool in this setting, for example, after radiofrequency thermal ablation (5), after lipiodol (131I) therapy (6), or in patients with unexplained rising serum AFP levels (7).
However, the sensitivity of 18F-FDG PET for detecting HCC is not better than that of conventional imaging (50%–70%) (8–11), mostly because well-differentiated HCC has a high rate of gluconeogenesis comparable with normal liver tissue, resulting in similar uptake of 18F-FDG (12). In contrast, high diagnostic performance has been reported with 18F-FDG for the detection of the other main primary liver malignancies—cholangiocarcinoma and hepatocholangiocarcinoma—or for liver metastases (13–16).
PET tracers of lipid metabolism have been proposed as a better method for the detection of HCC. 11C-labeled acetate was reported to be beneficial because of its better sensitivity, as high as 87%, for the detection of low- and intermediate-grade HCC (17). Choline is one of the components of phosphatidylcholine, an essential element of phospholipids in the cell membrane. Because of higher choline contents in HCC than in normal liver tissue (18,19), detected with magnetic resonance spectroscopy, we made the hypothesis that performance of PET/CT and 18F-fluorocholine, a choline analog, will be at least as good as that of 11C-acetate PET/CT. 18F-fluorocholine is more easily available and in a larger activity than 11C-acetate or 11C-choline in clinical PET centers; it provides higher image resolution thanks to its shorter positron length path. These arguments led us to perform a proof-of-concept study in 12 HCC patients, published in 2006 (20). It showed that 18F-fluorocholine was better than 18F-FDG at detecting HCC, with a trend to a more intense 18F-fluorocholine uptake in well-differentiated than in poorly differentiated HCC.
The present prospective phase III study was undertaken to compare the sensitivity of 18F-fluorocholine and 18F-FDG PET/CT for detecting HCC in patients with cirrhosis or chronic liver disease, characterize liver nodules detected by 1 of the standard imaging techniques—ultrasonography, spiral CT, MRI, or MR angiography (patients with a past history of HCC and newly discovered liver lesions were thus also eligible for lesion characterization and evaluation of extent), and restage the potential cancer in the case of significant uptake by one or several nodules. The secondary objective was to correlate 18F-fluorocholine and 18F-FDG uptake by any liver lesion with its differentiation.
MATERIALS AND METHODS
Methodology
This prospective study was accepted by the local ethics committee (CCPPRB) in December 2005 (Eudract 2006-000538-11). Patients gave their written informed consent. Each patient underwent both 18F-fluorocholine and 18F-FDG PET/CT examinations in a random order within 4 wk.
Our sample size of 34 patients with HCC was chosen because it could show a significant difference of 35% with regard to the sensitivity of 18F-fluorocholine versus 18F-FDG, with an α of 0.05 and a β of 0.10. Thus, patients were no longer included in the study when it was obvious that 34 of the patients already included had HCC.
After the last patient was recruited, but before the standard of truth (SOT) was determined, 18F-FDG and 18F-fluorocholine PET/CT results were read in a masked manner. Masked reading was performed for all PET (attenuation-corrected and noncorrected) and fused PET/CT images by 2 nuclear medicine specialists experienced in interpretation of both 18F-fluorocholine and 18F-FDG PET/CT who were not present in the department when the PET/CT images were acquired and did not meet the patients. 18F-fluorocholine and 18F-FDG PET/CT images were evaluated in a random order, different for the 2 tracers, during 2 different sessions separated by a 1-mo period. The evaluation of the likelihood of cancer for the whole patient and per-site was reported on a grid according to the following 5-grade scale: 0, no cancer or definitely nonpathologic aspect; 1, probably benign lesion; 2, equivocal lesion; 3, probably cancer; and 4, most probably cancer. Discrepant readings in a given set of images between the 2 masked readers were recorded by the clinical manager, and a consensus reading was organized. This consensus reading was used to determine diagnostic performance in relation to the SOT, whereas the 2 original grids were used for κ-measurement of agreement between the observers. For the determination of diagnostic performance, scores 0 and 1 were considered a negative result and levels 2–4 a positive one.
Follow-up data after the PET/CT scans were requested for all eligible patients. The data collected included results of histology, physical examination, medical imaging, and biologic assays at each visit during follow-up. The minimal follow-up period was 6 mo; when follow-up was shorter, in particular when the patient died during this period, the independent assessor decided for which sites the SOT could be determined.
On a per-patient basis, the SOT was HCC if any HCC lesion was histologically proven or if the Barcelona criteria were met (1). In this last case, determination of SOT consisted of either radiologic criteria (i.e., 2 coincident imaging techniques [of ultrasonography, spiral CT, MRI, and angiography] show a focal lesion > 2 cm with arterial hypervascularization) or combined criteria (1 imaging technique shows a focal lesion > 2 cm with arterial hypervascularization associated with AFP serum levels > 400 ng/mL).
On a per-site basis, the SOT for liver nodules was based only on histology performed on specimens obtained either after biopsy or after surgery. A maximum of 3 lesion sites was recorded for each of the 2 parts of the liver (right and left), corresponding to the 3 largest lesions with histologic evidence. The aim was to limit the number of liver sites per patient (maximum, 6) to avoid an excessive weight, in the per-site analysis, of patients with diffuse nodules. Furthermore, 18F-fluorocholine and 18F-FDG PET have a limited resolution and are not expected to detect and characterize nodules smaller than 5–7 mm.
Five extrahepatic sites were also defined prospectively: both lungs, peritoneum, skeleton, and other organs. Each of these organs counted for 1 site when the organ was suspected of being cancer-bearing (clinically or on any imaging modality). This option is widely used in evaluating imaging agents, to avoid a single patient with multiple metastases (e.g., in the lungs or the skeleton) having the same weight in the site-based evaluation as all other patients with fewer metastatic lesions. The SOT was determined in extrahepatic sites either on histology or on data of the 6-mo follow-up. When no such data permitted the assessment of the nature of the lesion, the site was excluded from analysis.
The SOT was determined, for each site in each patient, by an independent clinical assessor unaware of the results of both PET/CT examinations. This independent assessor was a hepatologist who did not participate in patient recruitment, PET/CT acquisitions, or masked reading.
By comparison, between the consensus masked reading and SOT in a given patient or in a given evaluable site, the result of 18F-fluorocholine and 18F-FDG examinations was determined to be true-positive (TP), true-negative, false-positive (FP), or false-negative (FN).
Sensitivity and specificity of 18F-fluorocholine and 18F-FDG PET/CT were then calculated and compared on a per-patient and per-site basis. Specificity was calculated as the rate of true-negative results in the patients who had no malignancy, on per-patient level, or in sites proven to be cancer-free, on per-site level.
Patients
Eighty-one patients were included and underwent both 18F-fluorocholine and 18F-FDG PET/CT studies from December 12, 2005, until September 19, 2008. Nine patients who had been included were considered noneligible by the independent assessor because they had no cirrhosis or chronic hepatic disease or were undergoing treatment. One patient had two 18F-fluorocholine and 18F-FDG PET/CT examinations. For this patient, both examinations were considered eligible and evaluated separately—the first examination for characterization and staging of liver lesions before any treatment and the second 16.5 mo later, after tumorectomy, because another nodule in the liver, suggesting recurrence, was detected.
PET/CT
Before both PET/CT studies, patients were instructed to fast for at least 6 h, except for diabetic patients, who had to fast for 4.5 h after taking their oral medication together with a meal. The 18F-fluorocholine used in this study (IASOcholine; Iason) had a scheduled activity of 4 MBq/kg of body mass.
18F-fluorocholine or 18F-FDG (5 MBq/kg of body mass) was administered intravenously in an infusion line connected to saline.
A Gemini Dual PET/CT camera (Philips) was used for imaging, with low-dose CT (120 kVp, 30–50 mAs) acquired first, followed by PET acquisition 10–20 min after 18F-fluorocholine injection or 60–90 min after 18F-FDG injection, covering a field of view from the skull to mid thighs.
Criteria for PET/CT Interpretation
The masked readers used the following criteria to describe malignancy. For both 18F-FDG and 18F-fluorocholine PET/CT studies, lesions were considered malignant if there were nonphysiologic foci of high uptake, unless the imaging context was evocative of benignity. For the 18F-fluorocholine PET/CT only, a lesion that appeared hypometabolic on 18F-fluorocholine images and of a tissue density on CT was considered malignant.
Statistical Analysis
The dedicated software Medcalc (version 10.2.0) was used to perform statistical analysis.
The sensitivity and specificity of 18F-FDG and 18F-fluorocholine PET/CT were compared using the McNemar test (2-tailed formulation, with a level of significance of 0.05).
Diagnostic performance between groups was compared using the Fisher exact test for qualitative variables (e.g., naïve vs. recurrent disease) and the Mann–Whitney test for quantitative variables (e.g., AFP).
Interreader variability and agreement were tested with weighted κ-statistics from the 2 independent masked readings of PET/CT images.
RESULTS
Evaluable Patients, Examinations, and Sites
The SOT could be determined by the independent assessor in 58 patients who underwent 59 18F-fluorocholine and 18F-FDG PET/CT examinations; we will refer to 59 cases in the patient-based analysis. In 14 other patients without histologic evidence and who did not meet the Barcelona criteria, the SOT could not be determined.
In 46 cases, patients had no past history of cancer. In 12 cases, patients had a past history of cancer previously considered as cured or in complete remission, but recently discovered liver nodules suggested recurrence: 8 HCC, 1 hepatocholangiocarcinoma, 1 cholangiocarcinoma, 1 colon cancer, and 1 prostate cancer. In 1 patient, colon cancer was diagnosed shortly after he had been included in the study to characterize an 80-mm liver nodule.
A total of 194 lesion sites were considered; the independent assessor was able to determine the SOT in 122 of them (113 hepatic and 9 extrahepatic sites). Lesion size was measured at postsurgical histology or on CT or MR images. The smallest diameter ranged between 0.4 and 15 cm (mean, 3.4 cm; median, 2.2 cm).
PET/CT Examinations
The actual injected activity of 18F-fluorocholine ranged between 2.9 and 4.6 MBq/kg of body mass. According to on-site and masked readers, a sufficient image quality was obtained with the minimal activity of 2.9 MBq/kg.
18F-fluorocholine and 18F-FDG PET/CT examinations of the same patient were performed in a random order: 18F-fluorocholine first in 31 patients (53%) and 18F-FDG first in 28 patients (47%, P = 0.8). The time between the 2 PET/CT examinations ranged from 1 to 24 d when 18F-fluorocholine was administered first and from 1 to 28 d when 18F-FDG was administered first, with an identical median of 4 d whether 18F-fluorocholine was performed first or second.
HCC
As determined by the protocol, the study included 34 patients with HCC. Histology revealed HCC in 27 patients and HCC with a contingent of cholangiocarcinoma (hepatocholangiocarcinoma) in 2 patients. In 5 patients, the Barcelona criteria (1) were met and considered as a surrogate for histologic diagnosis of HCC on a per-patient basis; however, no site-based evaluation could be done in those patients.
The patient-based sensitivity for the detection of HCC was 88% for 18F-fluorocholine and 68% for 18F-FDG (Table 1); the trend for 18F-fluorocholine superiority did not reach statistical significance (P = 0.07).
Diagnostic Performance of 18F-Fluorocholine and 18F-FDG PET/CT for Detection of HCC or Other Malignancies in Patients with Liver Nodules on Cirrhosis or Chronic Liver Disease
The site-based sensitivity for the detection of HCC or hepatocholangiocarcinoma was 84% for 18F-fluorocholine—significantly better than the 67% sensitivity for 18F-FDG (P < 0.01) (Table 1).
Subgroup Analysis of Sensitivity.
For each radiopharmaceutical, no significant difference in sensitivity between PET/CT performed in 27 naïve patients versus 7 patients with confirmed HCC recurrence was observed (85% vs. 100%, P = 0.6, for 18F-fluorocholine; 67% vs. 71%, P > 0.9, for 18F-FDG). In recurrent HCC, PET confirmed uptake by suggestive lesions in 5 patients with 18F-fluorocholine and in 3 of 5 with 18F-FDG; PET also showed an unexpected pulmonary lesion in 2 patients with both tracers.
As expected, AFP serum levels were greater in HCC patients than in other patients (P = 0.004). Eleven of the 34 patients with HCC or hepatocholangiocarcinoma had normal AFP levels (68% sensitivity for AFP). All of those 11 patients had TP 18F-fluorocholine results (100%) versus only 6 patients with TP 18F-FDG results (55%, P < 0.05); the 5 18F-FDG FN results corresponded to well-differentiated HCC in 3 patients but also to less-differentiated HCC or hepatocholangiocarcinoma in 2 patients.
In the subgroup of 11 patients with well-differentiated HCC, 4 had hot foci with both 18F-fluorocholine and 18F-FDG and 1 had hot 18F-FDG foci and a hypometabolic aspect of 1 lesion on 18F-fluorocholine (Fig. 1). Six of these patients had a positive result with 18F-fluorocholine only (Fig. 2), whereas 18F-FDG was never positive alone. On a per-patient basis, no FN result for 18F-fluorocholine PET/CT was observed; sensitivity was 100% for 18F-fluorocholine versus 45% for 18F-FDG (P < 0.003). Site-based sensitivity for the detection of the 32 well-differentiated HCC sites was 94% (30/32) for 18F-fluorocholine and 59% (19/32) for 18F-FDG (P = 0.001, Table 1). Only 2 sites were missed with 18F-fluorocholine, and they were also 18F-FDG–negative. There were 8 subcentimeter lesions (minimum diameter, <1 cm) of well-differentiated HCC, seven 18F-fluorocholine–positive (88%), and six 18F-FDG–positive (75%).
Maximum-intensity-projection images showing 2 liver foci; the smallest, in inferior part of liver, was 18F-fluorocholine– and 18F-FDG–positive. On transaxial slices of upper part of liver, center of largest lesion was photopenic on both 18F-fluorocholine (left) and 18F-FDG (right) PET/CT images and corresponded to hemorrhagic necrosis. At periphery of this lesion, there was definite 18F-FDG uptake, and 18F-fluorocholine was taken up but with lesser intensity than for noncancerous liver parenchyma (18F-fluorocholine tumor–to–non-tumor ratio T/NTR = 0.97). Postsurgical histology confirmed well-differentiated HCC in this part of lesion. Thus, as compared with nonmalignant liver, HCC tissue was hypermetabolic for 18F-FDG but hypometabolic for 18F-fluorocholine.
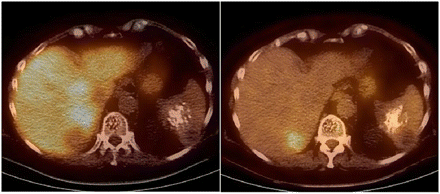
Maximum-intensity-projection images showing 2 liver foci, both hot on 18F-fluorocholine PET (left) but not visible on 18F-FDG PET (right) images. Both liver foci corresponded to untreated well-differentiated HCC.
In 18 other patients with intermediate or poorly differentiated HCC, or with hepatocholangiocarcinoma (which is considered to share the same poor prognosis), the difference of sensitivity (83% for 18F-fluorocholine vs. 78% for 18F-FDG) was not significant. Nine patients had foci of increased uptake on both PET/CT examinations, and 4 patients had foci of increased uptake on 18F-FDG PET/CT and hypometabolic lesions on 18F-fluorocholine PET/CT images (Fig. 3). In 3 patients, HCC could be detected with only 1 tracer—for 2 patients with 18F-fluorocholine and 1 with 18F-FDG. Two patients had FN results with both 18F-FDG and 18F-fluorocholine PET/CT: 1 patient with a single 2.2-cm liver lesion and 1 patient with hepatocholangiocarcinoma whose 2.1-cm liver lesion was missed but who had synchronous rectal cancer that was detected on 18F-FDG PET/CT. Site-based sensitivity for the detection of 38 less-differentiated HCC or hepatocholangiocarcinoma lesions was 76% (29/38) for 18F-fluorocholine and 74% (28/38) for 18F-FDG (P > 0.9, Table 1). There were 4 subcentimeter lesions: 3 were 18F-fluorocholine–positive and two 18F-FDG–positive. Concerning extrahepatic lesions, only 1 site of lung metastasis from hepatocholangiocarcinoma was evaluable with histology; both 18F-fluorocholine and 18F-FDG were taken up (Fig. 3).
Resection of left liver for hepatocholangiocarcinoma; 20-mm nodule subsequently developed in remaining liver (arrow). Nodule appeared hypometabolic for 18F-fluorocholine (bottom left) and avid for 18F-FDG (bottom right); hepatocholangiocarcinoma was confirmed by biopsy. On PET images of thorax (top left, 18F-fluorocholine; top right, 18F-FDG), widespread lung foci were discovered, corresponding to 1 metastasis but also to lesions of anthracosilicosis, and 1 thyroid nodule, which was benign.
In the case of HCC, a clear 18F-fluorocholine hypometabolic focus might favor less-differentiated cancers: only 1 of 5 liver HCC lesions appearing hypometabolic on fluorocholine PET/CT corresponded to well-differentiated HCC (Fig. 1).
Sensitivity of Combined 18F-Fluorocholine and 18F-FDG PET/CT Studies.
By associating 18F-fluorocholine– and 18F-FDG–positive PET/CT results, patient-based sensitivity for HCC or hepatocholangiocarcinoma (i.e., 18F-fluorocholine–positive or 18F-FDG–positive) was 94% (32/34)—2 HCC patients being 18F-fluorocholine–negative and 18F-FDG–positive. Two patients were negative on both PET/CT examinations; they had hepatocholangiocarcinoma or poorly differentiated HCC. On a per-site basis, fluorocholine or FDG PET/CT was positive for 63 of 70 (90%) HCC or hepatocholangiocarcinoma sites.
Other Malignancies
Ten patients had non-HCC malignancies.
Two patients had HCC, but a second cancer was demonstrated: 18F-FDG–positive rectal cancer and 18F-fluorocholine–positive lung metastases of prostate cancer.
In 4 patients, liver nodules corresponded to cholangiocarcinoma. Three were detected on both PET/CT examinations. Of 11 cholangiocarcinoma liver sites, 10 were detected with 18F-FDG versus 6 hot 18F-fluorocholine foci in a single patient, plus 2 hypometabolic lesions in other patients.
In 2 patients, a solitary liver metastasis of colon cancer took up 18F-FDG and was photopenic on 18F-fluorocholine PET/CT (Fig. 4); 1 patient also had a lung metastasis, which took up both tracers, 18F-FDG more avidly.
Transaxial liver slice of metastasis of colon cancer: photopenic on 18F-fluorocholine PET/CT (left) and hot on 18F-FDG PET/CT (right) images.
In 2 patients, lung cancer was discovered, positive with 18F-fluorocholine and 18F-FDG, whereas HCC recurrence was not proven.
Benign Conditions
Of 8 patients with all or some liver nodules corresponding to focal nodular hyperplasia (FNH), 7 (88%) had FP results on 18F-fluorocholine PET/CT (Fig. 5). Of 8 patients with pure adenoma, 1 had a FP result with 18F-fluorocholine. In 1 patient, cholangitis resulted in another FP result with 18F-fluorocholine. Thus, 18F-FDG specificity was significantly better than 18F-fluorocholine specificity (Table 1).
Maximum intensity projection in case of FNH: 1 liver lesion positive on 18F-fluorocholine PET (left) and not visible on 18F-FDG PET (right) images.
Three extrahepatic lesions were histologically proven to be benign. Colonic polyps in 1 patient did not take up 18F-fluorocholine or 18F-FDG. In contrast, an anthracosilicotic lesion and an oncocytic adenoma of the thyroid gland in another patient gave FP results with both 18F-fluorocholine and 18F-FDG (Fig. 3).
Specificity of Combination of 18F-Fluorocholine and 18F-FDG.
Of all 17 patients with benign liver lesions, 18F-fluorocholine and 18F-FDG were both FP in only 1 patient with FNH and adenomatous lesions, but the only lesion that took up 18F-FDG was 18F-fluorocholine–negative. Thus, none of the 31 benign liver sites corresponded to a FP result with both radiopharmaceuticals.
Reproducibility of Reading Between Masked Readers
On a per-patient basis, concordance between the 2 masked readers (using the 5-grade scale) was κ= 0.76 for 18F-fluorocholine and 0.88 for 18F-FDG. On a per-site basis, the corresponding values were 0.71 and 0.88, respectively.
DISCUSSION
Limitations of Study
This study is a phase III trial designed according to the guidelines of the European Medicine Agency. Nevertheless, the study has some limitations. Patients were recruited by several centers, but PET/CT studies were all performed in Hôpital Tenon, where the 18F-fluorocholine was delivered, permitting a homogeneous quality of imaging but hampering the demonstration of the reproducibility of the results in another institution. The number of HCC patients (34) in this study limits the power of the tests in subgroup analysis, although the superiority of 18F-fluorocholine sensitivity reached statistical significance in the subgroup of 11 patients with well-differentiated HCC. Because 1 recruiting center is a referral center when FNH is suspected, the proportion of FNH among benign lesions was larger than in other series: 47% versus 30% (P < 0.05) in a recent series of 573 liver nodules (21). Because FNH was responsible for FP 18F-fluorocholine results, this particular recruitment reduced specificity and made the calculation of accuracy and predictive values meaningless for other centers. Another limitation is the lack of hypothesis to explain that all 18F-fluorocholine photopenic–hypometabolic tissue lesions were of a malignant nature. Normal hepatocytes are rather 18F-fluorocholine–avid, as illustrated by the background in normal liver tissue, definitely higher than with 18F-FDG. The presence of cells that are less 18F-fluorocholine–avid than normal hepatocytes is clear in the case of colorectal metastasis. But there is no clear explanation for this loss of choline transport in some HCC lesions. Furthermore, in the same patient, 1 hypermetabolic lesion and 1 hypometabolic lesion on 18F-fluorocholine PET/CT both corresponded to well-differentiated HCC (Fig. 1). In our series, no benign lesion showed this hypometabolic 18F-fluorocholine pattern. Although the authors did not mention this fact, a 11C-choline photopenic and 18F-FDG–positive liver lesion of poorly differentiated HCC was identified by Yamamoto et al. (22).
Sensitivity for Liver Lesions
The main result of the current study was that 18F-fluorocholine was more sensitive than 18F-FDG for the detection of HCC sites in patients with liver nodules on cirrhosis or a chronic hepatic disease or with a high likelihood of HCC. In 2006, we reported a better detection rate for 18F-fluorocholine than for 18F-FDG in 12 patients with known HCC (20)—that is, a sample with a different recruitment. Better results with 18F-fluorocholine in the case of well-differentiated HCC were also suggested and confirmed in the present series. Since then, this indication of 18F-fluorocholine imaging has been reported, using nonhybrid PET, in only 1 case of recurrent multifocal HCC (23).
In the present study, the sensitivity of 18F-FDG PET/CT for detection of intrahepatic HCC was 68% on per-patient analysis, in the upper part of the range of published values (close to 70% reported by Delbeke et al. (8) or 64% by Wudel et al. (24)).
As we expected when designing the study, the diagnostic performance of 18F-fluorocholine for the detection of intrahepatic HCC was close to published values for 11C-acetate, with a sensitivity ranging from 75% to 87% (18,25). Using 11C-choline PET and considering only foci with increased uptake as positive, Yamamoto et al. (22) recently reported a lesion-based sensitivity of 63% only. If one limits in the present study the TP 18F-fluorocholine results to foci of increased uptake, as Yamamoto et al. (22) did, overall patient-based sensitivity would be 74% for 18F-fluorocholine and 68% for 18F-FDG; overall site-based sensitivity would be 76% for 18F-fluorocholine and 67% for 18F-FDG. In well-differentiated HCC sites, the difference of sensitivity (91% for 18F-fluorocholine vs. 59% for 18F-FDG) would still be statistically significant (P = 0.05).
The present study confirms a significantly better sensitivity for 18F-fluorocholine in the case of well-differentiated HCC (94% vs. 59% for 18F-FDG) but not in the case of poorly differentiated HCC or of hepatocholangiocarcinoma. The relationship between uptake of lipid tracer and HCC differentiation was already mentioned with 11C-acetate (17,25) and 11C-choline (22).
In HCC with normal AFP levels, which is most frequently well-differentiated, 18F-fluorocholine PET/CT seems interesting because 18F-fluorocholine sensitivity was independent of AFP levels, contrary to the sensitivity of 18F-FDG.
Even though 18F-fluorocholine was more sensitive than 18F-FDG for detecting liver lesions of well-differentiated HCC, it was also taken up by less differentiated lesions. Thus, none of the tracers is suited for an accurate noninvasive individual determination of HCC lesion differentiation.
In the present study, of 11 cholangiocarcinoma lesions, 5 took up fluorocholine and 2 showed hypometabolism with 18F-fluorocholine; 18F-FDG was taken up by 8, more intensely than was 18F-fluorocholine. The observed uptake of a lipid tracer by cholangiocarcinoma is in accordance with the results of Park et al. (25): a 69% (9/13) detection rate for 11C-acetate versus 100% for 18F-FDG. However, in a previous study, none of the 3 cholangiocarcinoma patients was 11C-acetate PET–positive (17).
Detection of Extrahepatic Lesions
Concerning PET detection of distant metastases of HCC, a trend for a better sensitivity for 18F-FDG than for 11C-acetate has been reported, but diagnostic performance improved by performing PET/CT examinations with both radiopharmaceuticals (25,26). In the present study, there was only one histologically proven metastasis of HCC, and no conclusion can be drawn. This low prevalence of HCC distant metastases in our series is probably due to the early stage of potential HCC in the eligible patients. Second cancers were more frequent, with a better contrast on 18F-FDG than on 18F-fluorocholine PET/CT, except for a lung metastasis of prostate cancer, which is not surprising because 18F-fluorocholine is a far better tracer than 18F-FDG for prostate cancer (27).
Synergy of 18F-Fluorocholine and 18F-FDG in This Context
In the present study, the combination of 18F-fluorocholine and 18F-FDG PET/CT examinations increased the detection rate of liver HCC sites: from 84% with 18F-fluorocholine and 67% with 18F-FDG to 90% for both. Furthermore, 18F-FDG and 18F-fluorocholine appeared complementary for the detection of extrahepatic malignant sites, second primary cancers in particular. Our results also favor a dual-tracer approach, imaging separately glucose and lipid metabolisms (25,26).
Specificity
In the present study, 18F-fluorocholine and 18F-FDG PET/CT were positive in some inflammatory lesions; but 18F-fluorocholine was also positive in most FNH patients, whereas 18F-FDG PET/CT was negative in all 8 FNH patients. The good specificity of 18F-FDG PET in the case of FNH was reported in 2000 by Kurtaran et al. (28), although a possible 18F-FDG uptake has also been described (29). 11C-acetate is taken up by FNH, as observed by Ho et al. (17). Magini et al. recently reported that in patients selected for having proven or suspected benign liver lesions 34 of 36 (94%) FNH lesions took up 11C-acetate (30); in contrast, only 3 lesions (8%) in 1 patient showed an increased 18F-FDG uptake. However, FNH lesions were characterized in most patients (25/31 [80%]) by noninvasive imaging before PET/CT. In the present study, 18F-fluorocholine uptake was observed in a similar proportion of FNH patients (88%). These authors also reported uptake of both 18F-FDG and 11C-acetate in 4 of 5 lesions of hepatic adenoma (80%). This uptake does not correspond to our results: none of the 10 sites with pure adenoma took up 18F-FDG and only 1 took up 18F-fluorocholine.
In our series, tissue lesions appearing hypometabolic for 18F-fluorocholine were all malignant. Lesions of a benign origin appearing photopenic on 11C-acetate PET have been reported by Ho et al. (17), but they were due to hemangiomas and the nontissue content could be seen on CT.
CONCLUSION
This prospective study confirmed that 18F-fluorocholine PET/CT was able to detect HCC in liver nodules, even of subcentimeter size, and demonstrated that 18F-fluorocholine is more sensitive than 18F-FDG for detecting well-differentiated HCC. In contrast, the sensitivity of 18F-fluorocholine and 18F-FDG PET/CT was not significantly different in the case of less differentiated HCC.
18F-FDG PET/CT can be proposed first in the case of uncharacterized liver nodules because 18F-FDG was more frequently taken up by liver malignancies other than HCC and was less frequently taken up by FNH.
18F-fluorocholine PET/CT appeared to be a more effective modality for staging and recurrence evaluation of HCC, in particular the well-differentiated forms, and thus an alternative to 11C-acetate PET/CT, with a better yield for tracer production and routine use and simpler logistics.
The combination of both tracers had overall better performance than 18F-fluorocholine or 18F-FDG alone and seems useful in that it misses neither clusters of well-differentiated HCC nor distant metastases.
Acknowledgments
We greatly acknowledge the efforts of the team of technologists of the nuclear medicine department of Hôpital Tenon for performing the PET/CT examinations. We thank the physicians who referred patients for inclusion into the study and reported their follow-up data, the histopathologists whose work was fundamental for the SOT, and Dr. J. Buscombe, who edited the manuscript.
- © 2010 by Society of Nuclear Medicine
REFERENCES
- Received for publication January 26, 2010.
- Accepted for publication August 9, 2010.