Abstract
The aim of this SPECT study was to investigate the effects of donepezil on regional cerebral blood flow (rCBF) in patients with mild to moderate Alzheimer’s disease (AD) using statistical parametric mapping. Methods: rCBF was noninvasively measured using 99mTc-ethyl cysteinate dimer in 35 AD patients with a Mini-Mental State Examination score > 16 on initial evaluation. Baseline and follow-up SPECT studies with a mean interval of 12 mo were performed on these patients. We used the adjusted rCBF images in the relative flow distribution (normalization of global cerebral blood flow for each patient to 50 mL/100 g/min with proportional scaling) to compare these groups through statistical parametric mapping. Results: In the follow-up study, the adjusted rCBF was significantly preserved in the right and left anterior cingulate gyri, right middle temporal gyrus, right inferior parietal lobules, and prefrontal cortex of donepezil-treated AD patients, compared with placebo-treated AD patients. Conclusion: Treatment with donepezil for 1 y appears to reduce the decline in rCBF, suggesting preservation of functional brain activity.
Donepezil hydrochloride (Aricept; Eisai Co., Tokyo, Japan) is a piperidine-based acetylcholinesterase inhibitor that is used clinically for the symptomatic treatment of mild to moderate Alzheimer’s disease (AD) (1,2). Donepezil has been shown to be well tolerated and, compared with placebo, to significantly improve cognition and to help maintain global function (2). The results of 24-wk studies have indicated that the well-established benefits of donepezil on cognition may extend to improved performance of complex activities of daily living (1). However, the impact of treatment with donepezil, in comparison with placebo, on regional cerebral blood flow (rCBF) has not been reported in patients with mild to moderate AD. The aim of this study was to evaluate this impact.
MATERIALS AND METHODS
Subjects
Thirty-five patients (22 men, 13 women; age range, 57–80 y; mean age, 70.4 y) who complained of memory impairment were recruited from an outpatient memory clinic of the National Center Hospital for Mental, Nervous, and Muscular Disorders, National Center of Neurology and Psychiatry, between 1998 and 1999. The clinical diagnosis of AD was based on the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV) (3), and criteria of the National Institute of Neurological and Communicative Disorders and Stroke and the Alzheimer’s Disease and Related Disorders Association (4). The patients had a Mini-Mental State Examination score > 16. Dementia was staged using the clinical dementia rating and ranged from 0.5 to 2. The patients were randomized to receive daily oral doses of either donepezil (5 mg/d) or placebo. Each patient underwent baseline SPECT and MRI at the time of the initial evaluation and was clinically followed by a second SPECT and MRI examination after 12 mo. Demographic information is shown in Table 1.
Patient Characteristics
A subset of neuropsychological tests from the standard clinical protocol was selected a priori (Table 2) to profile multiple cognitive abilities, including frontal lobe functioning, memory, visuospatial ability, and language skills. Raven’s Coloured Progressive Matrices (5) was used to measure visuoperceptual, attentional, and problem-solving skills. In addition, the digit span task (5) was used to measure attention. The learning of a list of 10 words and story recall (5) were used to measure short-term verbal memory and language ability. The Ray-Osterrieth complex figure test (5) was used to evaluate both visuoconstructional ability and visual memory. Measures of frontal lobe functioning included the Stroop test (6). Impaired performance on the Stroop test is common in patients with frontal lobe lesions and is also associated with a specific pattern of rCBF in the anterior cingulate cortex (7). All tests were administered and scored according to standard protocols. Age- and education-adjusted norms were applied when available. No focal brain lesions on MRI were observed except for age-related hyperintensities on T2-weighted images.
Neuropsychological Test Results
Global and Regional CBF Measurements
Before SPECT scanning was performed, an intravenous line was established in the right cubital vein of all patients while they were supine with their eyes closed in a dimly lit, quiet room. Each patient received a 600-MBq intravenous injection of 99mTc-ethyl cysteinate dimer (ECD). The global cerebral blood flow (CBF) was noninvasively measured using graphic analysis as described in previous reports, without any blood sampling (8–10). Passage of the tracer from the aortic arch to the brain was monitored in a 128 × 128 format for a short period of less than 2 min at 1-s intervals using the rectangular gamma camera of a triple-head SPECT system (MULTISPECT 3; CTI, Knoxville, TN/Siemens Medical Systems, Inc., Hoffman Estates, IL) equipped with high-resolution fanbeam collimators. Regions of interest were hand-drawn over the aortic arch and both brain hemispheres. The hemispheric brain perfusion index was determined before the start of the initial backdiffusion of the tracer from brain to blood using the method described for a previous study (8):
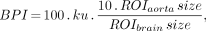 Eq. 1
where BPI is the brain perfusion index, ROIaorta is the region of interest drawn over the aortic arch, ROIbrain is the region of interest drawn over the brain, and ku is the unidirectional influx rate of the tracer from blood to brain, as determined by the slope of the line in graphic analysis within the first 30 s after injection. Then, brain perfusion index (x) was converted to global CBF values (y) obtained by 133Xe-inhalation SPECT studies (y = 2.60x + 19.8) (8).
Eq. 1
where BPI is the brain perfusion index, ROIaorta is the region of interest drawn over the aortic arch, ROIbrain is the region of interest drawn over the brain, and ku is the unidirectional influx rate of the tracer from blood to brain, as determined by the slope of the line in graphic analysis within the first 30 s after injection. Then, brain perfusion index (x) was converted to global CBF values (y) obtained by 133Xe-inhalation SPECT studies (y = 2.60x + 19.8) (8).
Ten minutes after the injection of 99mTc-ECD, brain SPECT imaging was performed. The projection data were obtained in a 128 × 128 format for 24 angles in 120° for each camera with 50 s per angle. A Shepp and Logan filter was used for SPECT image reconstruction at 0.75 cycle per centimeter. Attenuation correction was performed using Chang’s method. The following linearization algorithm (11) of a curve-linear relationship between brain activity and blood flow was applied to calculate rCBF and to correct for incomplete retention of 99mTc-ECD in the brain:
 Eq. 2
where Fi and Fr represent CBF values for region i and a reference region, respectively, and Ci and Cr are the SPECT counts for region i and a reference region, respectively. The cerebral hemisphere was used as the reference region, and global CBF obtained from graphic analysis was substituted for the CBF value for the reference region. The linearization factor α was set at 2.59, which was the value proposed by Friberg et al. (11).
Eq. 2
where Fi and Fr represent CBF values for region i and a reference region, respectively, and Ci and Cr are the SPECT counts for region i and a reference region, respectively. The cerebral hemisphere was used as the reference region, and global CBF obtained from graphic analysis was substituted for the CBF value for the reference region. The linearization factor α was set at 2.59, which was the value proposed by Friberg et al. (11).
Image Formatting
All subsequent image manipulation and data analysis were performed on a Windows 98– (Microsoft, Redmond, WA) and UNIX-based computer. The software for image manipulation included MATLAB (versions 4.2c and 5.3; The MathWorks, Inc., Natick, MA) and the statistical parametric mapping (SPM) programs SPM99 and SPM96 (Wellcome Department of Cognitive Neurology, London, U.K.). Transverse-slice image volumes were made compatible with SPM by the creation of usable headers for the images. Briefly, for each image a file was created that contained data on image size, number of slices, pixel depth (16-bit), maximum pixel value, and voxel size. All brain-slice images were then sampled and averaged to arrive at a mean pixel intensity value for that image. The intensity threshold was set at 80% of the whole-brain mean. The images were then spatially normalized in SPM99 to a standardized stereotactic space based on the atlas of Talairach and Tournoux (12), using 12-parameter linear and also 8 nonlinear iteration algorithms. Because the distribution of 99mTc-ECD in the brain is different from that of 15O-water, which was used as the PET template in the SPM program, we used an original 99mTc-ECD template, which was prepared in a previous study (13), for spatial normalization. The normalization routine also included further isotropic smoothing, to a total of 12 mm. This corresponded to almost twice the resolution (7.6-mm full width at half maximum) of the SPECT scanner. The initial parameters of the image were 128 × 128 × n, where n is the number of slices. The final image format was 16-bit, with a size of 79 × 95 × 68 and a voxel size of 2 × 2 × 2 mm.
Image Analysis
Data analysis was performed using SPM96. Statistical parametric maps are spatially extended statistical processes that were used to characterize regional specific effects in imaging data. SPM combines the general linear model and theory of gaussian fields (14,15) to make statistical inferences about regional effects. Regional CBF differences were examined as an intergroup comparison between the donepezil-treated and placebo-treated groups at baseline and in the follow-up SPECT study and as an intragroup comparison between the baseline and follow-up SPECT studies in each group. Moreover, to examine images for specific regions of differences in time-course perfusion changes from the baseline to the follow-up SPECT studies between donepezil-treated and placebo-treated patients, we compared 2 studies using 2 contrasts with study interaction (multistudy and multisubject with different conditions in SPM96). The first and second contrasts examined areas of elevation and decline, respectively, of time-course perfusion changes in the donepezil-treated patients compared with placebo-treated patients. The analysis was performed with or without the implication of changes in global CBF levels as a confounding covariate. The gray matter threshold was set at 0.8. We first used raw data (absolute rCBF parametric maps without global normalization) and then adjusted rCBF images (normalization of global CBF for each subject to 50 mL/100 g/min with proportional scaling) to compare relative rCBF distribution in 2 groups. The resulting set of values for comparison constituted a statistical parametric map of the statistic SPM{t}. The SPM{t} maps were then transformed to the unit of normal distribution (SPM{z}) and reached a threshold at P = 0.001. The resulting regions were examined using multiple comparisons. The significance of each region was estimated at a threshold of P = 0.05 using distributional approximations from the theory of gaussian fields (15). These areas of significance were best seen when overlaid on a normalized MR image to obtain a clear view of the location of the perfusion changes.
RESULTS
Neuropsychological Profile
Table 2 shows the mean scores of the donepezil- and placebo-treated patients on the neuropsychological tests. Compared with the donepezil-treated patients, the placebo-treated patients performed significantly worse on both forward digits (P = 0.0007) and reverse digits (P = 0.03) in the digit span test, on 30-min delayed recall in the Ray-Osterrieth complex figure test (P = 0.02), and on word-dot in the Stroop test (P = 0.01) in the follow-up SPECT study. For the placebo-treated patients, mean scores for the Mini-Mental State Examination (P = 0.003), forward digits in the digit span test (P = 0.01), and word-dot in the Stroop test (P = 0.03) declined significantly in the follow-up SPECT study. In contrast, no significant differences (P > 0.05) were found for the other neuropsychological tests.
Global Effects
The baseline and follow-up global CBF values were 35.9 ± 3.4 and 35.5 ± 2.7 mL/100 g/min, respectively, in donepezil-treated patients and 36.8 ± 4.3 and 35.4 ± 4.4 mL/100 g/min, respectively, in placebo-treated patients. There was neither a group × time interaction effect nor a main effect (group or time) on global CBF in a 2 × 2 (donepezil/placebo × baseline/follow-up) repeated-measures ANOVA.
Regional Effects
Adjusted rCBF.
Figure 1 and Table 3 show the voxels that had a significant decline in adjusted rCBF during the follow-up period among placebo-treated patients compared with donepezil-treated patients (no voxel significantly increased). Significant group × time interaction effects were observed in time-course rCBF changes between placebo-treated and donepezil-treated patients. Compared with the donepezil-treated patients, the placebo-treated patients showed a significant rCBF decline in the right and left anterior cingulate gyri, right prefrontal cortex, right inferior parietal lobules, and right middle temporal gyrus. Significant differences in adjusted rCBF were not found between the 2 groups at baseline and in the follow-up SPECT study, nor were significant differences present between the baseline and follow-up SPECT studies in either intragroup comparison.
Adjusted rCBF decrease in placebo-treated AD patients compared with donepezil-treated AD patients and from baseline study to follow-up study as shown by SPM. Significant decrease is seen as maximal intensity projection (MIP) and colored areas superimposed on standard 3-dimensional anatomic template.
Location and Peaks of Significant (P < 0.001) Decreases in Adjusted rCBF
Absolute rCBF.
No significant time-course changes in absolute rCBF were seen between donepezil-treated and placebo-treated AD patients. In addition, significant differences in absolute rCBF were not observed between the 2 groups at baseline and in the follow-up SPECT study or between the baseline and follow-up SPECT studies in either patient group.
DISCUSSION
To our knowledge, only 2 studies have investigated the impact of donepezil on rCBF. One study reported that the global cerebral metabolic rate for glucose in donepezil-treated AD patients did not significantly change from the baseline rate, and this finding agrees well with our result. However, that study did not investigate regional cerebral metabolic rate (16). In another study, using 99mTc-hexamethylpropyleneamine oxime SPECT with SPM analysis, Warren et al. (17) found a significant decrease in adjusted rCBF in the left parietal lobe and both cingulate gyri in donepezil-treated AD patients. However, unlike our study, their study was not placebo controlled.
We found preservation of adjusted rCBF in the anterior cingulate gyrus and right prefrontal cortex of donepezil-treated patients. With regard to this finding, several previous studies of functional neuroimaging have described the anterior cingulate cortex as being a site that subserves the execution of action, motivation, planning, initiation of action, and anticipation (18–22). A PET study observed the effect of physostigmine, an acetylcholinesterase inhibitor, on working memory efficiency and also found increased rCBF in the right prefrontal cortex (23). These studies support the validity of our SPECT findings.
Although the principal mechanism by which donepezil probably benefits patients with AD is poorly understood, an explanation by Kilgard and Merzenich (24) may help clarify the mechanism. They explored the mechanisms that allow the cortex to selectively improve the neural representations of behaviorally important stimuli while ignoring irrelevant ones. The results of their study suggest that acetylcholine potentiates the formation of new neural connections, that is, both declarative and procedural new memories. The pathology of AD that accounts for progressive dementia is primarily the loss of neural connectivity. These connections form morphologic bases for knowledge. On the other hand, in a brain affected by AD, neuronal loss is observed in the nucleus basalis, the nucleus of the diagonal band of Broca, and the medial septal nuclei. The neuronal loss impairs the formation of new memories. Therefore, patients with AD have progressive loss of old memories and impaired ability to partially compensate the loss by reforming memories. In this conceptualization, donepezil potentiates the formation of new neural connections (hence memories) and sequentially compensates for the loss of neural connectivity caused by the disease. Finally, SPM allowed us to handle images and analyze data reliably and objectively and permits complicated analyses that can improve interstudy variability caused by the analytic process itself.
CONCLUSION
Treatment with donepezil for 1 y appeared to reduce the decline in rCBF, suggesting preservation of functional brain activity in donepezil-treated patients. This study suggested that SPM may be useful in assessing the response to donepezil therapy in AD patients.
Footnotes
Received Dec. 29, 2000; revision accepted Jun. 11, 2001.
For correspondence or reprints contact: Seigo Nakano, MD, Department of Geriatric Medicine, National Center Hospital for Mental, Nervous, and Muscular Disorders, 4-1-1, Ogawahigashi, Kodaira, Tokyo 187-8551, Japan.







