Visual Abstract
Abstract
In recent decades, researchers worldwide have directed their efforts toward enhancing the quality of PET imaging. The detection sensitivity and image resolution of conventional PET scanners with a short axial field of view have been constrained, leading to a suboptimal signal-to-noise ratio. The advent of long-axial-field-of-view PET scanners, exemplified by the uEXPLORER system, marked a significant advancement. Total-body PET imaging possesses an extensive scan range of 194 cm and an ultrahigh detection sensitivity, and it has emerged as a promising avenue for improving image quality while reducing the administered radioactivity dose and shortening acquisition times. In this review, we elucidate the application of the uEXPLORER system at the Sun Yat-sen University Cancer Center, including the disease distribution, patient selection workflow, scanning protocol, and several enhanced clinical applications, along with encountered challenges. We anticipate that this review will provide insights into routine clinical practice and ultimately improve patient care.
PET/CT is a noninvasive examination that plays a crucial role in the visual assessment of tumor staging, restaging, and therapy response monitoring (1). Researchers worldwide have been focused on improving the sensitivity and resolution of PET imaging. In the case of the short axial field of view (SAFOV), PET detectors capture approximately only 1% of emitted coincidence photons. Because of the limitation posed by the length of the AFOV, whole-body scanning has traditionally entailed shifting the bed (typically from head to thigh in about 5 bed positions). Each bed position requires about 1–3 min, resulting in whole-body scans taking 10–30 min.
Extending the AFOV length could increase the number of detectors and allow the simultaneous capture of more emitted photons. The image quality could be improved through enhanced counting statistics achieved by broader solid angle coverage. The 194-cm long-AFOV (LAFOV) PET scanner, uEXPLORER (United Imaging Healthcare), became operational in 2018 (2). Given its substantially higher sensitivity (3–5) and a long scan range in a single bed position, total-body PET can provide ultrahigh system sensitivity, coupled with high spatial resolution. There are several enhanced clinical applications using total-body PET/CT: full-body coverage, short acquisition time, low injection dose, high image quality, and simultaneous total-body dynamic imaging. Despite these advantages, the high detection of both anatomic and pathologic uptake could still lead to challenging and equivocal interpretations in clinical practice (6). Therefore, experimental experience is crucial for understanding and use.
In this review, we provide an overview of the application of the uEXPLORER system in Sun Yat-sen University Cancer Center (SYSUCC), including the patient characteristics, selection workflow, scanning protocol, several enhanced clinical applications, and challenges encountered in routine clinical practice. Future perspectives on the opportunities offered by total-body PET scanners are also presented.
APPLICATION OF TOTAL-BODY PET/CT IN SYSUCC
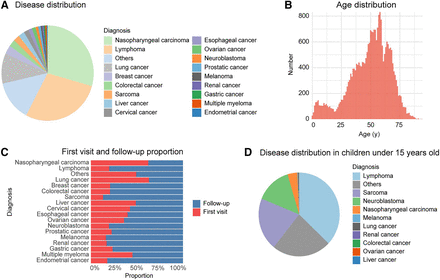
From May 1, 2020, to June 30, 2023, 30,786 examinations were conducted in our center using the uEXPLORER PET/CT scanner. The top 3 cancers were nasopharyngeal carcinoma (NPC), lymphoma, and lung cancer (Fig. 1A). Among these, NPC and lung cancer accounted for a significant proportion of initial diagnoses; other diseases were more prevalent in follow-up examinations (Fig. 1C). Most patients were in the age range of 45–65 y (Fig. 1B). Numerous pediatric patients were younger than 15 y, primarily diagnosed with lymphoma, sarcoma, neuroblastoma, and NPC (Fig. 1D). In clinical practice, there was also a small proportion of nononcologic patients, such as patients with unexplained elevated tumor markers but no malignant imaging signs or those with suspected lesions of malignancy that were finally pathologically diagnosed as benign diseases.
Application of total-body PET/CT at SYSUCC. (A) Disease distribution. (B) Age distribution. (C) Disease stage distribution. (D) Disease distribution in pediatric patients younger than 15 y. Others = nononcologic patients or patients who have not yet been followed up.
SCANNING PROTOCOLS OF TOTAL-BODY PET/CT IN SYSUCC
Compared with SAFOV PET/CT, a LAFOV PET/CT scanner faces new challenges in acquisition workflow. Attenuation correction in PET/CT hybrid imaging typically relies on CT transmission data. Although there have been studies focused on using lutetium background radiation for attenuation correction (7,8), no precedent has been established in clinical practice. This means that CT acquisition must fully cover the range of the 194-cm PET detectors. PET/CT examinations include different types of CT scans depending on their purpose, such as a low-dose CT scan solely for attenuation correction and/or a diagnostic CT scan with a higher x-ray dose (1). Therefore, 2 routines of CT scans can be used in the workflow of the uEXPLORER scanner: total-body diagnostic CT scan with a higher x-ray dose (TB routine) and total-body, low-dose CT scan for attenuation correction plus a torso (from skull to mid thigh) diagnostic CT scan with a higher x-ray dose (torso routine).
By considering different ages and diseases, we have designed various acquisition protocols. For adults older than 20 y, TB routine or torso routine was selected on the basis of doctors’ recommendations and depending on the risk of systemic metastasis. Furthermore, certain parameter modifications or optimizations were based on the TB and torso routine (Table 1; Supplemental Table 1 [supplemental materials are available at http://jnm.snmjournals.org]) to create specific disease protocols. For example, high-resolution PET reconstruction of the head was added for patients with lung cancer; fast, deep inspiration breath-hold PET acquisition was performed for patients with poor alignment of thoracoabdominal lesions; and a short acquisition time was performed for NPC patients while meeting the diagnostic requirements for the high throughput of patients in our center.
Key Parameters of Total-Body PET/CT Acquisition Protocols at SYSUCC
To enhance the number of lines of response for pairs of annihilation photons by increasing the axial acceptance angle (9), it becomes necessary for the CT acquisition range to extend beyond the PET reconstruction range if a limited-area tumor imaging is required. With regard to dose accumulation, we performed a diagnostic CT scan with a higher x-ray dose in the limited area and included an additional 10 cm of low-dose CT scan for attenuation correction above and below.
For children, adolescents, and young adults (≤20 y), the dose of the TB routine is notably lower than that of the torso routine, leading us to use the TB routine exclusively. The pixel size for PET reconstruction decreases in descending order according to age. With regard to CT scanning and reconstruction parameters, the acquisition protocols for each age group are divided into 3 categories: nonlymphoma, lymphoma, and lymphoma follow-up, with decreasing CT doses. For children aged 7 y and younger with smaller body sizes, we use a tube voltage of 100 kV, whereas for those older than 7 y, we use 120 kV, both with automatic tube current modulation. In addition, in pediatric patients younger than 15 y, we use a rotation time of 0.3 s and reduce the CT acquisition time from 25 to 15 s to minimize the risk of body motion. Besides, for youths with larger body sizes (weight > 45 kg), we appropriately reduce the reference milliampere·seconds because of the higher dose administrated when using tube current modulation. Moreover, all protocols for youths have incorporated thin-slice CT reconstructed with the artificial intelligence iterative reconstruction algorithm.
SELECTION OF PET/CT SCANNERS ACCORDING TO PATIENTS’ MEDICAL CONDITIONS
The selection among various PET/CT devices is a critical aspect of the daily operations at SYSUCC. The facility uses a total-body PET/CT scanner (uEXPLORER) alongside 2 SAFOV PET/CT scanners: uMI 780 (United Imaging Healthcare) and Biograph mCT.X (Siemens Healthineers). To ensure patient interests are prioritized, medical considerations are accurate, and scanning processes are efficient, physicians are tasked with making numerous decisions to determine which patients are suitable for total-body PET/CT examinations. The workflow is detailed in Figure 2.
Selection of PET/CT devices according to patients’ medical conditions at SYSUCC.
For newly diagnosed patients, total-body PET/CT is given priority for the following conditions: pediatric patients, for whom total-body PET/CT aided in reducing the PET imaging agent dosage (10–12) and potentially shortening acquisition time; patients who required whole-body scanning, such as those with systemic diseases, malignances affecting multiple organs or extremities, and advanced tumors with distant metastases beyond the scanning range of SAFOV PET/CT; patients with complex diseases or occult lesions, for whom total-body PET/CT might enhance diagnosis sensitivity (13,14) and assist in recognizing the tumor’s origin (15); patients experiencing untenable body position induced by cancer-related pain or involuntary movement (16); and patients expected to undergo multiple PET/CT examinations as part of regular surveillance, for whom total-body PET/CT also aided in reducing cumulative radiation exposure.
For patients undergoing dynamic PET/CT examinations or participating in the pharmacokinetics studies involving radiopharmaceuticals, total-body PET/CT is the primary choice. In the case of patients returning for reevaluation of therapeutic efficacy by comparing current and previous PET/CT images, it is important to ensure that the same device is used for the current scanning as for the previous scan. This consistency is necessary to maintain comparable detection sensitivity and SUV. For patients with claustrophobia exhibiting as fear of enclosed spaces, SAFOV scanners are the primary recommendation.
ADVANTAGES AND APPLICATIONS OF TOTAL-BODY PET SCANNING
Low-Dose Injection of PET Imaging Agents
The high sensitivity, along with a dramatically improved signal-to-noise ratio, of the total-body PET/CT scanner allows a substantial reduction in the injected radiopharmaceutical activity. There have been several studies exploring total-body PET/CT with low-dose injection activity (as summarized in Table 2). Most of these studies have focused on adult populations. However, children and young adults are more sensitive to the stochastic effects of ionizing radiation, because their bodies are undergoing rapid cell division. In addition, children and adolescents have a relatively longer life span and more time to manifest potential radiation effects than adults (17,18). Concerns about additional radiation have arisen as a result of multiple PET/CT scans performed before and during treatment.
Clinical Studies Exploring Low-Dose Injection Activity of 18F-FDG Using Total-Body PET/CT
To minimize unnecessary ionizing radiation exposure, the injection dose of 18F-FDG for pediatric patients younger than 15 y at our center is 1.85 MBq/kg, with a standard scanning time of 10 min. Our research (12) has found that total-body PET/CT with a one-half dose of 18F-FDG (1.85 MBq/kg; estimated whole-body effective dose, 1.76–2.57 mSv) demonstrates excellent performance in pediatric patients, providing adequate image quality and lesion conspicuity. Theoretically, image quality could be maintained at ultralow doses, going as low as a one-tenth dose (0.37 MBq/kg) of the standard dose when using a total-body PET scanner (11). A study of 18F-FDG dose deescalation and shortened acquisition duration using total-body PET/CT is under implementation at our center, and we aim to formulate a standardized working protocol of pediatric tumor imaging. An example of a pediatric patient scanned with a one-fifth dose (0.74 MBq/kg) of the standard dose of 18F-FDG using total-body PET/CT with an acquisition time of 10 min is shown in Figure 3.
Example of total-body PET scan with one-fifth dose of 18F-FDG (0.79 MBq/kg) activity. Thirteen-year-old boy diagnosed with relapsed anaplastic lymphoma kinase-positive anaplastic large cell lymphoma underwent ninth PET/CT examination for follow-up after chemotherapy. Slight radiotracer uptake of inflammatory lymph node in right neck (arrow) is shown. Axial PET image of liver displays good background quality.
Low-Dose CT in Total-Body PET/CT
Dosimetric studies related to total-body PET/CT have mainly focused on reducing the 18F-FDG injection dose, with fewer studies dedicated to the CT dose. The CT dose constitutes a significant portion of the total effective dose, especially when low or ultralow 18F-FDG injection doses are achieved (11,19,20). In our previous study, the potential of total-body PET/CT to reduce the CT dose in pretreatment pediatric patients with lymphoma was validated (21). Through optimization of scanning parameters, it was possible to reduce the CT dose in a total-body PET/CT examination to an average of approximately 4 mSv, representing a 66.1% reduction in the CT dose compared with SAFOV PET/CT. This study primarily focused on the diagnosis and staging of pretreatment pediatric patients. Exploring lower CT doses for various clinical applications, such as treatment evaluation, is a worthwhile endeavor. Pediatric lymphoma of various subtypes is almost always 18F-FDG-avid and relies more on PET than CT for diagnosis and staging, so further investigation is needed to understand the universal potential of total-body PET/CT in reducing the CT dose across various tumors. In addition, the CT component dose in PET/CT scans should be institution-specific and determined according to its local preferences, scanner hardware, technologist experience, and available reconstruction methods (22). Besides, an ongoing study at our center exploring the diagnostic efficacy of low-dose CT combined with low-dose 18F-FDG activity is being implemented (Fig. 4). Preliminary results suggest that the total effective dose of a total-body PET/CT scan can be reduced to a minimum of 3.75 mSv.
Comparison of image quality between CT-Dx and LDCT combined with different 18F-FDG injection doses. Five-year-old boy diagnosed with B-cell lymphoblastic leukemia underwent complete remission after systemic chemotherapy. Total-body PET/CT examinations were performed during 2-y follow-up. One-half dose of 18F-FDG (1.85 MBq/kg) combined with reduced CT dose was used for latter examination (right) to minimize radiation exposure. Representative CT images, axial PET images, and fused PET/CT images of liver show diagnostic and staging efficacy is not compromised. CT-Dx = diagnostic CT scan with higher x-ray dose; LDCT = low-dose CT.
Rapid Scanning
The ultrahigh system sensitivity and spatial resolution of the total-body PET scanner offer an opportunity to reduce acquisition time while preserving image quality. In certain scenarios, shorter scan durations can serve as a salvage method to mitigate or eliminate the requirement for sedation or repeat scanning, which is particularly advantageous in the context of pediatric imaging among children from 3 to 5 y old (23). However, for infants and toddlers, involuntary movement might occur during the initial phase of the scan, resulting in imperceptible mismatch at the beginning. Therefore, younger pediatric patients still require sedation or must remain asleep during the scan. In addition, a shorter imaging duration can benefit patients with advanced tumors who may encounter respiratory difficulties or discomfort while reclining on the scanner bed (16). For patients with brain metastases who exhibit compulsive movements, the total-body PET scanner can provide relief during the examination or potentially shorten the duration of constraints. Although it is rare, the necessity for PET imaging in such circumstances should not be underestimated.
How fast could the total-body PET scan be? Prior investigations have demonstrated that the acquisition times can be reduced to 30 s when using the uEXPLORER scanner for total-body PET/CT (20,24). The rapid PET protocol, with acquisition durations of 30–45 s, can deliver image quality equivalent to that of SAFOV digital uMI 780 PET/CT, which typically necessitates 2–3 min per bed position. The 30-s total-body PET/CT achieved similar sensitivities and accuracy in a cohort of 88 oncology patients, thus adequately meeting clinical diagnostic requirements, to those achieved with the standard 300-s acquisition time (25). Similar results were supported by another LAFOV scanner, the Biograph Vision Quadra PET/CT (Siemens Healthineers). Shorter LAFOV acquisitions of 30 s were sufficient for target lesion identification, suggesting that ultrafast or low-dose acquisitions can be considered acceptable (26).
Full-Body Coverage
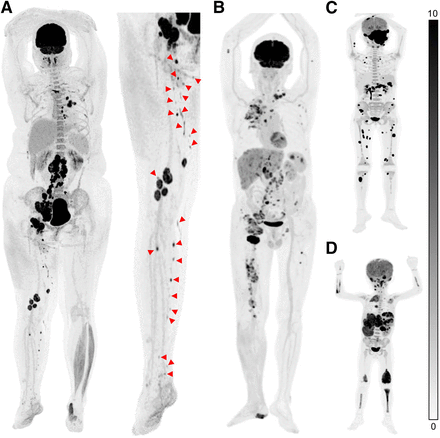
SAFOV PET imaging protocols recommend scanning from the base of the skull to the upper-mid-thigh region, considering that most bone metastases tend to migrate to the red bone marrow, predominantly located in axial and appendicular skeletons (27). Besides, a comprehensive scan of the lower extremities using the SAFOV modality increases the number of bed positions required, thus extending the scan time. Unfortunately, this protocol overlooks the presence of widespread musculoskeletal metastases. Pediatric and adult-onset malignancies such as cutaneous lymphoma, renal cancer, lung cancer, and melanoma have a higher propensity of developing distant metastases in the extremities (28). Moreover, several cancers are characterized by biologic heterogeneity, with substantial variations observed both within the primary site and among metastatic sites. This inevitably leads to diagnostic and classification inaccuracies when based on a limited number of biopsy samples, resulting in deviations in therapeutic selection and response to treatment. Although SAFOV PET/CT may not fully reflect overall disease activity, total-body PET offers the potential to detect cancers at multiple sites, with full-body coverage achievable in a single bed position (Fig. 5). This greatly enhances the sensitivity for detecting various cancers and holds the potential for a groundbreaking impact on comprehensive disease management. Despite limited experience, total-body PET allows the assessment of nononcologic systematic diseases, including degenerative atherosclerosis (29,30), rheumatoid arthritis, and deep venous thrombosis (31).
Advantages of full-body coverage using 18F-FDG (3.7 MBq/kg) total-body PET/CT. (A) PET images of 32-y-old man diagnosed with right pelvic melanoma and lymph nodal metastases. Arrowheads indicate venous thrombosis within right leg veins. (B) 65-y-old man with malignant melanoma. Diagnostic accuracy of total-body PET was confirmed by extensive detection of cutaneous primary lesion and lymph node metastases throughout entire body. (C) 9-y-old girl with lymphoma revealed by biopsy of left orbital mass. Total-body PET image shows lymph, cutaneous, and bone involvement. (D) 3-y-old child diagnosed with B lymphoblastic lymphoma. Multiple masses in liver, spleen, and kidney with multisystem invasion, including lymph nodes and focal distal bones, were detected.
High Sensitivity and Improved Lesion Detection
Increased sensitivity broadens the applicability of total-body PET scans across various clinical scenarios, especially those that were unattainable with SAFOV PET/CT scanners (Fig. 6). A cohort study featuring a head-to-head intraindividual comparison between 2 PET scans demonstrated that total-body PET/CT exhibited greater sensitivity in detecting small lesions (4.3 mm; SUVmax, 1.0) or those with low uptake (tumor-to-liver ratio, 1.6; SUVmax, 4.1) (32). This heightened sensitivity yields a superior signal-to-noise ratio, enabling the identification of small, low-contrast tumor deposits and micrometastases (33). Another study illustrated that total-body PET achieves enhanced contrast recovery through the use of small voxels, coupled with low background variability. This enhancement aids in the detection and quantification of uptake in smaller lesions (5). Furthermore, artifacts stemming from respiratory, cardiac, intestinal motion, and overall body movement can greatly affect the image quality. However, the elevated sensitivity affords the opportunity for total-body scans to be conducted in a single breath-hold, as well as for motion-frozen scans of the heart, lungs, and gastrointestinal tract (4).
Example of intraindividual comparison of 18F-FDG (3.7 MBq/kg) total-body PET (left) and conventional PET (right) images in lung cancer patient with liver and bone metastases. Axial PET images of total-body PET scans revealed 4 lesions in liver (arrows) that were barely recognizable in conventional PET/CT scans, because radiotracer uptake of liver lesions was similar to physiologic liver background.
Whole-Body Dynamic Studies
In conjunction with tracer kinetic modeling, whole-body dynamic 18F-FDG PET enables a multiparametric quantification approach that holds the potential to unveil clinical insights into the metabolic properties of tissues. In turn, these derived-image metrics offer the possibility to facilitate tumor characterization and assess therapy responses (34,35). Our current emphasis is on patients with NPC or lung cancer, both prevalent diseases in our center. We initiate a 60-min dynamic scan after the injection of 3.0 MBq/kg 18F-FDG. To ensure a seamless workflow, patients scheduled for dynamic scans undergo their initial examination each day. The intricate tumor structure of NPC led us to develop a segmentation method based on parametric images from dynamic PET/CT, which improves the accuracy of cancer segmentation (36). For lung cancer, we have shown that the kinetic parameter of Ki obtained from whole-body dynamic PET/CT provides a more precise characterization of the metabolic heterogeneity of non–small cell lung cancer. This metric correlates with levels of immune cell infiltration and the response to immune-chemotherapies (37).
However, parametric imaging does encounter challenges related to extended scanning times. Encouragingly, several studies have explored shorter acquisition protocols to enhance clinical efficiency (38–40). Another study demonstrated the feasibility of performing accurate 18F-FDG Patlak analysis using a scaled population-based input function with only 20 min of PET data from a LAFOV PET scanner (41).
CHALLENGES OF TOTAL-BODY PET/CT
False Positives Arising from High Sensitivity
The enhanced image quality also introduces challenges in terms of image interpretation and differentiation. In clinical practice, the primary challenge associated with total-body PET/CT scanners lies in distinguishing between benign and malignant lesions. Unlike the mild-to-moderate 18F-FDG uptake observed in the spinal cord in SAFOV PET/CT scans, total-body PET/CT scans have shown intense 18F-FDG uptake in the distal spinal cord, which is considered a physiologic phenomenon (42). Structures previously deemed non–18F-FDG-avid in SAFOV PET/CT scans, such as the gallbladder, may exhibit FDG uptake (43). This necessitates careful consideration when distinguishing these findings from inflammatory or neoplastic diseases. The emerging pattern observed in total-body PET/CT scans warrants further exploration and summarization.
Because of improved spatial resolution, total-body PET/CT images reveal a greater number of small lesions and lymph nodes. However, we should not automatically assume that all of these are malignant. Radiologists face challenges in determining the nature of these findings (Fig. 7). Taking biopsy samples for every lesion or lymph node in most patients is impractical. Therefore, it is crucial to approach the interpretation of these small lesions and lymph nodes with great caution to avoid false-positive diagnoses. Limited studies specifically address false-positive rates in total-body PET/CT scans, and this issue has been recognized as a potential challenge associated with total-body PET/CT scanners (44,45).
Example of retroperitoneal small lymph nodes posing challenges for diagnosis. Fifty-five-year-old woman with surgically removed cervical cancer was referred for 18F-FDG (3.7 MBq/kg) total-body PET/CT scan. Axial PET images and fused PET/CT images display paraaortic small lymph nodes (arrowheads) with higher 18F-FDG uptake (SUVmax, 6.5) than physiologic background.
Claustrophobia and Other Psychologic Disorders
Claustrophobia, anxiety, and other unanticipated psychologic disorders during imaging examinations not only result in excessive motion, which precludes diagnostic-quality imaging, but also can negatively affect the patient experience. The LAFOV scanner, with larger body coverage, tends to induce more stress in patients than SAFOV scanners. Patients with claustrophobia at our center found SAFOV scanners to be more acceptable in practice, even if they required a longer acquisition time. Therefore, SAFOV scanners were the primary recommendation for patients with claustrophobia. Most patients with mild or moderate anxiety or claustrophobia can complete the scan with the aid of psychologic support and audiovisual intervention performed before and during imaging by the referred physicians and technicians.
However, PET imaging remains challenging for patients with severe claustrophobia. Several interventions and efforts, such as open PET geometry (46), quieter machines, guided imagery, fragrance administration, and patient positioning devices (47), should be developed to minimize these psychologic disorders. This not only improves patient satisfaction but also enhances operational efficiency and diagnostic capacity.
Differences Between Total-Body and SAFOV PET/CT Systems Introduce Challenges in Harmonization
The obvious differences among devices pose hurdles for the quantitative assessment of PET parameters in therapeutic evaluation and disease monitoring, because these parameters are substantially influenced by the specific PET scanners in use. Furthermore, the employment of different filtering and reconstruction methods across various PET scanners can exacerbate the variability among PET parameters (48). In the clinical setting of SYSUCC, which is equipped with multiple PET devices, the major strategy for ensuring parameter comparability involves using the same devices for both baseline and follow-up scans, as described in an earlier section. An additional crucial strategy involves standardizing patient preparation, image acquisition and reconstruction, and image interpretation, aligning these with recommendations from the European Association of Nuclear Medicine (1) and the Uniform Protocols in Clinical Trials (49). The third strategy entails conducting annual quality control experiments using a set of phantoms for adherence to the European Association Research Ltd. accreditation program (50). Furthermore, comparable PET parameters can be achieved through the application of advanced reconstruction algorithms, such as the point spread function (51), Bayesian penalized likelihood reconstruction (52), and EQ.PET software (Siemens) (53). Nevertheless, when multiple centers are involved, harmonization becomes a more complicated endeavor because of the challenges of ensuring standardized patient preparation and scan procedures. In addition, the feasibility of advanced reconstructions may be limited by the absence or lack of raw data. It is imperative to promote harmonization strategies in the routine operations of multiple medical institutions.
SUMMARY
Total-body PET/CT scanners have demonstrated their superiority over SAFOV systems in terms of sensitivity and lesion detection. They offer the advantages of requiring less administered radioactivity and shorter acquisition times. This increase in signal collection efficiency has enabled the optimization of clinical routines and the enhancement of several applications in disease diagnosis, staging, treatment response evaluation, and prognosis. Nevertheless, concerns inevitably arise about the ultimate benefits of total-body PET imaging. Many challenges, including false positives, psychologic disorders, and harmonizing results among different devices, must be addressed. In our center, we have reviewed the advantages and clinical applications of the total-body PET scanner, highlighting the substantial advancements made in various fields. Our aim is that this article will provide insights into routine clinical practices and ultimately improve patient care. We believe that there are still many impactful applications to be developed, and further demonstrations of the capabilities of the uEXPLORER scanner are warranted.
DISCLOSURE
Yumo Zhao is an employee of United Imaging Healthcare Co. Ltd. No other potential conflict of interest relevant to this article was reported.
ACKNOWLEDGMENTS
We thank our industry advisors at United Imaging Healthcare Co. Ltd. for their expertise and assistance.
- © 2024 by the Society of Nuclear Medicine and Molecular Imaging.
REFERENCES
- Received for publication October 25, 2023.
- Revision received January 31, 2024.