Visual Abstract
Abstract
Nectin cell adhesion molecule 4 (nectin-4) is a transmembrane protein overexpressed on a variety of cancers and plays an important role in oncogenic and metastatic processes. The nectin-4–targeted antibody–drug conjugate enfortumab vedotin has been approved for treating locally advanced or metastatic urothelial cancer, but the efficacy in other types of cancer remains to be explored. The aim of this study was to evaluate the feasibility of nectin-4–targeted PET imaging with 68Ga-N188 as a noninvasive method to quantify membranous nectin-4 expression in multiple tumor types—an approach that may provide insight for patient stratification and treatment selection. Methods: Sixty-two patients with 16 types of cancer underwent head-to-head 68Ga-N188 and 18F-FDG PET/CT imaging for initial staging or detection of recurrence and metastases. Correlation between lesion SUVmax and nectin-4 expression determined by immunohistochemistry staining was analyzed in 36 of 62 patients. Results: The SUVmax of 68Ga-N188 had a positive correlation with membranous nectin-4 expression in the various tumor types tested (r = 0.458; P = 0.005), whereas no association was observed between the SUVmax and cytoplasmic nectin-4 expression. The detection rates for patient-based analysis of 68Ga-N188 and 18F-FDG PET/CT examinations were comparable (95.00% [57/60] vs. 93.33% [56/60]). In patients with pancreatic cancer, 68Ga-N188 exhibited a potential advantage for detecting residual or locally recurrent tumors; this advantage may assist in clinical decision-making. Conclusion: The correlation between nectin-4–targeted 68Ga-N188 PET imaging and membranous nectin-4 expression indicates the potential of 68Ga-N188 as an effective tool for selecting patients who may benefit from enfortumab vedotin treatment. The PET imaging results provided evidence to explore nectin-4–targeted therapy in a variety of tumors. 68Ga-N188 may improve the restaging of pancreatic cancer but requires further evaluation in a powered, prospective setting.
Nectin cell adhesion molecule 4 (nectin-4), formerly known as poliovirus receptor–like 4, is a type I transmembrane protein and belongs to the nectin family of immunoglobulinlike adhesion molecules (1,2). Overexpression of nectin-4 has been identified in a variety of cancers, including bladder, breast, ovarian, and pancreatic tumors. Enfortumab vedotin (EV), an antibody–drug conjugate targeting nectin-4, was recently approved by the U.S. Food and Drug Administration and the European Medical Agency for its efficacy in treating locally advanced or metastatic urothelial cancer (UC) (3,4). The efficacy of EV in other solid tumors remains to be explored. The presence of nectin-4 expression has been observed both on cell membranes and in the cytoplasm of tumor cells, with the membrane expression as the biologic premise for EV binding to achieve therapeutic effects (1,5). A recent study showed that nectin-4 membranous expression decreases with disease progression and that low nectin-4 expression in metastatic biopsies may indicate resistance to EV (6). A shorter progression-free survival for patients who have metastatic tumors with lower nectin-4 expression after receiving EV was also reported (6). There is a potential urgent need for noninvasive methods to identify patients with high levels of membranous nectin-4 expression for personalized EV therapy.
Recently, we developed a nectin-4–targeted radiotracer, 68Ga-N188, for PET/CT imaging of nectin-4 expression (7). Such agents can overcome the limitations of biopsy for detecting heterogeneous biomarker expression and achieve dynamic assessment of nectin-4 expression noninvasively. In the pilot study, 68Ga-N188 detected nectin-4-expressing lesions in 14 UC patients, with a positive correlation between lesion SUVmax and nectin-4 expression determined by immunohistochemical (IHC) staining.
Besides UC, nectin-4 expression has been reported in multiple other types of cancer, and it is of interest to explore 68Ga-N188 PET in those tumor types. Such investigation may help to further validate this imaging method in clinical settings and provide insight on nectin-4 expression patterns across different cancer types—information that could help to promote the application of EV in treating cancers other than UC. We initiated this study and explored the utility of nectin-4–targeted PET in 16 types of primary tumors and recurrent/metastatic relapses in 62 patients. Nectin-4 expression in the tumors of 36 patients was determined with IHC. We further evaluated the correlation between SUVmax of PET imaging and membranous nectin-4 expression. The initial results are presented in a head-to-head comparison with 18F-FDG PET and suggest that 68Ga-N188 can be an effective method for detecting cancer and quantifying membranous nectin-4 expression.
MATERIALS AND METHODS
Patients
This prospective, single-center study was conducted at Peking University First Hospital and was approved by the institutional review board (approval number 2022-301). The study was registered at ClinicalTrials.gov (NCT05593107). All participants were consecutively recruited for enrollment from August to December 2022 and provided written informed consent.
Inclusion criteria were as follows: adult patients aged 18 y and older, patients who had pathologically confirmed cancer or for whom cancer was highly suspected and patients who were referred for cancer restaging, patients who underwent both 18F-FDG and 68Ga-N188 PET/CT scans within 1 wk, and patients who agreed to be followed up for pathologic and imaging results. Exclusion criteria were as follows: female patients who were pregnant or lactating, female patients who planned to become pregnant within 6 mo, and patients who were unable to provide written informed consent.
Radiopharmaceutical Preparation
18F-FDG was provided by Atom High-Tech Co., Ltd. 68Ga-N188 was prepared according to a previously reported procedure (7). Briefly, 3 mL of 0.05 M HCl solution containing [68Ga]GaCl3 (518–1,665 MBq), 200 μL of 1.0 M sodium acetate, and 100 μg of N188 were heated at 90°C for 10 min. After being cooled to room temperature, the solution was extracted with an activated Sep-Pak Light C18 Cartridge (Waters Corp.), and 68Ga-N188 was eluted with 0.6 mL of 80% ethanol aqueous solution. After purification and sterilization, 308–962 MBq of 68Ga-N188 could be obtained with a radiochemical yield of 53.9% ± 5.9% (mean ± SD) (not decay corrected, n = 30) and a radiochemical purity of greater than 99%, as analyzed by radio–high-performance liquid chromatography.
PET/CT Imaging Protocol
All participants fasted for 6 h before 18F-FDG PET/CT examination and 18F-FDG was administered intravenously (range of 3.70–5.18 MBq/kg) at serum glucose levels of less than 130 mg/dL. No special preparation was required before 68Ga-N188 PET/CT imaging. The injected dose for 68Ga-N188 was 2.22–2.96 MBq/kg. PET/CT acquisitions (uMI780; United Imaging Health Care) were performed 40–60 min after injection from head to midthigh. Low-dose, noncontrast CT (tube voltage of 120 kV, tube current of 100 mA/s, and matrix of 512 × 512) was performed for attenuation correction and anatomic reference. A total of 4 or 5 PET bed positions were acquired with a matrix of 192 × 192 and 1.5 min/bed position. All PET data were reconstructed using the ordered-subset expectation maximization algorithm with 2 iterations and 20 subsets. 18F-FDG and 68Ga-N188 PET/CT images were analyzed at a postprocessing workstation (uExceed, version R001; United Imaging Health Care).
PET/CT Imaging Analysis
Both 18F-FDG and 68Ga-N188 PET/CT images were reviewed and evaluated independently by 2 accredited nuclear medicine physicians with more than 10 y of experience. Any disagreement was resolved by comprehensive discussion for consensus. The lesions were considered visually positive when the focal accumulation of 68Ga-N188 and 18F-FDG was higher than that in adjacent background tissue, except for suspected physiologic or benign radiotracer uptake. For 68Ga-N188 imaging, physiologic radioactivity was expected in the kidneys and bladder. The numbers of lymph nodes and distant metastases were counted and recorded, with a maximum of 10 lesions recorded within a given organ or region. For semiquantitative analysis, the SUVmax normalized to body weight was automatically calculated and recorded for each lesion.
Histopathologic results were used as the gold standard for the final diagnosis. Follow-up clinical and conventional imaging examinations (ultrasound, CT, or MRI) for at least 3 mo were considered the reference standard for participants without pathologic results. Tumor TNM stage was determined with the TNM classification system (8) for patients undergoing initial staging.
IHC Staining
Sections of paraffin-embedded tumor specimens were obtained from surgically resected tumor or biopsy specimens. Samples were dewaxed and pretreated with antigen retrieval solution (1:50 dilution; ZSGB-Bio Inc.) for 20 min in a microwave oven. Samples were stained for nectin-4 (1:1,000 dilution; ab189514; Abcam) and detected using a ZSGB-BioDAB IHC Detection Kit (ZSGB-Bio Inc.). The proportion of stained cells was captured with MShot Digital Imaging System V9.0 (MShot).
The IHC results for nectin-4 expression were independently reviewed by 2 pathologists who were blinded to the clinical information and PET/CT imaging results. Both visualized staining intensity and proportion of positive tumor cells were evaluated. Both cytoplasmic nectin-4 expression and membranous nectin-4 expression were scored. The final nectin-4 expression staining was scored as 4 categories: 0 (no expression), 1 (weak expression), 2 (moderate expression), and 3 (strong expression).
Statistical Analysis
All statistics were analyzed using SPSS software (version 27.0.1; IBM Corp.). Quantitative data were presented as the mean ± SD. The lesion detection rates of 68Ga-N188 and 18F-FDG PET/CT imaging were compared using the McNemar test. The differences in tumor radioactivity between the 2 imaging examinations were evaluated using the paired-sample t test or the Wilcoxon signed rank test. The Spearman rank correlation coefficient was applied for the correlation between lesions’ SUVmax and nectin-4 expression. A P value of less than 0.05 was considered statistically significant.
RESULTS
Participant Characteristics
Participant characteristics are shown in Table 1. The flow chart of the study is presented in Figure 1. Sixty-two patients (29 female and 33 male) were included, with a median age of 62 (range, 32–86), and underwent paired 68Ga-N188 and 18F-FDG PET/CT examinations. A total of 16 types of malignancies were included, with the majority being pancreatic cancer (n = 19; 30.6%) and urothelial carcinoma (n = 18; 29.0%), followed by ovarian cancer (n = 6; 9.7%) and cervical cancer (n = 3; 4.8%).
Summary of Participant Characteristics
Flowchart of participant enrollment and study design.
All patients tolerated 68Ga-N188 and 18F-FDG well, and no adverse effects were reported. Among these patients, 40 of 62 were included for initial staging, and the diagnosis was histologically confirmed. The other 22 patients had a prior history of tumors and underwent PET/CT for restaging of tumor recurrence and metastases. Detailed information about the 62 enrolled patients is given in Supplemental Table 1 (supplemental materials are available at http://jnm.snmjournals.org).
Imaging Manifestations of 68Ga-N188 in Various Tumors
The average SUVmax of 68Ga-N188 PET/CT in the different tumor types (60/62 participants) is shown in Figure 2, and the representative maximum-intensity-projection images from 68Ga-N188 PET/CT imaging are displayed in Supplemental Figure 1. One patient (patient 41) who presented for UC initial staging was excluded because the postoperative pathology was negative while the biopsy pathology was positive. Considering clinical and pathologic results, the small tumor might be removed with the initial biopsy. In another UC patient (patient 49), there was no tumor local recurrence or distant metastasis after surgery, chemotherapy, and immunotherapy, and he was also excluded.
Average SUVmax of 68Ga-N188 PET/CT in 60 cases of 16 tumor types.
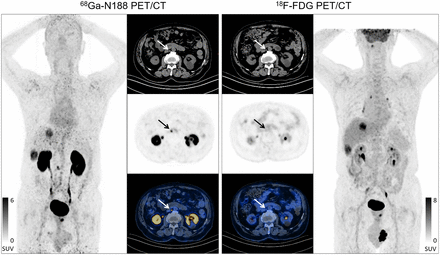
As shown in Figure 2, the highest average SUVmax was observed in intrahepatic cholangiocarcinoma, urothelial carcinoma, and cutaneous squamous cell carcinoma, followed by cervical cancer, esophageal cancer, endometrial carcinoma and pancreatic cancer. Representative images from a patient (patient 52) with intrahepatic cholangiocarcinoma are shown in Figure 3. The primary liver lesions were identified clearly by both 68Ga-N188 and 18F-FDG PET/CT imaging.
68Ga-N188 PET/CT and 18F-FDG PET/CT images in 59-y-old woman with intrahepatic cholangiocarcinoma before treatment (patient 52). Lesions were clearly visible on both maximum-intensity-projection images. Axial CT images showed large lesions in right liver lobe with heterogeneously increased radioactivity of 68Ga-N188 (SUVmax, 5.2) and 18F-FDG (SUVmax, 15.9) on axial PET and PET/CT fusion images (arrows).
Correlations of SUVmax and Nectin-4 Expression
The nectin-4 expression of primary and metastasis samples from 36 patients was evaluated and is shown in Supplemental Figure 2 and Supplemental Table 2. A distinct positive correlation was evident between SUVmax and membranous nectin-4 expression (r = 0.458; P = 0.005), as shown in Figure 4, whereas no significant correlation was found between SUVmax and cytoplasmic nectin-4 expression (r = 0.033; P = 0.847). Figure 5 shows representative correlated images of 68Ga-N188 PET/CT (patient 21 vs. patient 61) and IHC staining (upper row: membranous nectin-4 expression with a score of 3; lower row: membranous nectin-4 expression with a score of 0).
Correlation between membranous nectin-4 expression and SUVmax.
Correlation of 68Ga-N188 PET/CT images and IHC staining. (A) 68Ga-N188 PET/CT images in 86-y-old man with urothelial carcinoma (patient 21). Left renal pelvis and proximal ureter tumors (arrows) showed substantial radioactivity from 68Ga-N188 (SUVmax, 4.8) and strong membranous nectin-4 expression (score, 3). (B) 68Ga-N188 PET/CT images in 39-y-old man with hepatocellular carcinoma (patient 61). Large tumor in right liver lobe (arrows) showed low and diffuse radioactivity of 68Ga-N188 (SUVmax, 2.1). Negative membranous nectin-4 expression (score, 0) but moderate cytoplasmic nectin-4 expression (score, 2) were observed on IHC image.
Comparison of Detection Rates
The imaged cohort included the 40 patients who presented for initial staging and the other 22 patients who presented for restaging, as described earlier. Forty-five primary tumors were identified in the 40 patients undergoing initial staging (1 patient with urothelial carcinoma and 1 patient with intrahepatic cholangiocarcinoma had multiple presumed primary tumors), and 12 residual/local recurrent tumors were identified in the 22 patients undergoing restaging. Overall, 93 lymph nodes and 63 distant metastases were detected across all cases. The patient-based and lesion-based comparison results for rates of detection of 68Ga-N188 and 18F-FDG PET/CT are shown in Table 2.
Rates of Detection of 68Ga-N188 and 18F-FDG for Patient-Based and Lesion-Based Comparisons
For the patient-based comparison, 68Ga-N188 and 18F-FDG PET/CT examinations yielded comparable detection rates (95.00% [57/60] vs. 93.33% [56/60]), with no statistical difference. For 2 participants, the results obtained over follow-up were confirmed to be true-negative results, as mentioned earlier (participants 41 and 49).
For lesion-based comparison, 18F-FDG detected all 45 primary tumors (100.00% [45/45]), and 68Ga-N188 identified 39 primary tumors (86.67% [39/45]) (P = 0.031). The 6 tumors missed by 68Ga-N188 belonged to 4 types, including urothelial carcinoma, intrahepatic cholangiocarcinoma, cervical cancer, and hepatocellular carcinoma.
For the 12 residual and locally recurrent tumors, 68Ga-N188 detected all 12 tumors (100.00% [12/12]), but 18F-FDG failed to identify 4 of them (66.67% [8/12]) (P = 0.125). Interestingly, these 4 lesions were all pancreatic tumors in patients undergoing restaging, including 1 with postoperative recurrence (patient 18) and 3 with residual lesions after adjuvant chemotherapy (patients 19, 29, and 30), and the results were further confirmed by follow-up imaging. Representative images from a patient with pancreatic cancer after treatment (patient 29) are shown in Figure 6. A residual tumor in the head and uncinate process of the pancreas with increased 68Ga-N188 accumulation but no 18F-FDG uptake was noted.
68Ga-N188 PET/CT and 18F-FDG PET/CT images in 62-y-old man with pancreatic cancer after chemotherapy plus radiotherapy (patient 29). Axial CT images showed soft-tissue mass in head and uncinate process of pancreas (arrows). Mass with increased radioactivity of 68Ga-N188 (SUVmax, 3.4) was observed on axial PET and PET/CT fusion images. In contrast, no abnormal 18F-FDG uptake of mass (SUVmax, 3.2) was noted on corresponding PET/CT images (arrows).
Among the 104 lymph nodes detected by 68Ga-N188 and 18F-FDG PET/CT examinations, 93 positive and 11 negative lymph nodes were defined according to the pathologic and follow-up results. 18F-FDG PET/CT demonstrated higher detection rates than 68Ga-N188 PET/CT (94.62% [88/93] vs. 76.34% [71/93] [P < 0.001] for lymph node metastases and 96.83% [61/63] vs. 71.43% [45/63] [P < 0.001] for distant metastases).
The sensitivity and accuracy of 18F-FDG PET/CT were higher than those of 68Ga-N188 PET/CT (P < 0.001 and P = 0.002, respectively), and 68Ga-N188 PET/CT exhibited higher specificity than 18F-FDG PET/CT (63.64% [7/11] vs. 36.36% [4/11]), although differences did not reach statistical significance (P = 0.250) (Table 3).
Diagnosis of Lymph Node Involvement by 68Ga-N188 and 18F-FDG
Changes in Tumor Staging and Management
In 58 of 62 patients, agreement on TNM staging or restaging was obtained on the basis of 68Ga-N188 and 18F-FDG PET/CT. The staging or restaging of 2 patients (patients 1 and 59) with pancreatic cancer was upstaged by 68Ga-N188 PET/CT compared with 18F-FDG PET/CT for 1 metastatic peripancreatic node (N1 misjudged as N0) (patient 59) and multiple liver metastases (M1 misjudged as M0) (patient 1) recorded as negative by 18F-FDG PET/CT (Supplemental Fig. 3). In contrast, 18F-FDG PET/CT upstaged 1 patient with intrahepatic cholangiocarcinoma (patient 8) and 1 patient with ovarian cancer (patient 47). For the former, 68Ga-N188 detected only 1 of the liver lesions and missed involvement in multiple regional lymph nodes (T1bN0M0 for 68Ga-N188 vs. T2N1M0 for 18F-FDG). For the latter, 1 metastatic lymph node in the right cardiophrenic angle was staged as M1 by 18F-FDG examination but missed by 68Ga-N188 PET/CT imaging.
Of note is that 68Ga-N188 detected 2 paraaortic lymph node metastases in 1 patient with bladder cancer (patient 10) that were negative on 18F-FDG PET/CT imaging (Fig. 7). Because of the presence of liver metastasis, the TNM stage of this patient was not changed by the additional finding. However, the identification of additional lesions may provide complementary value for therapeutic decision-making in clinical practice.
68Ga-N188 PET/CT and 18F-FDG PET/CT images in 84-y-old man with urothelial carcinoma after treatment with transurethral resection of bladder tumor plus pelvic radiotherapy (patient 10). Metastatic liver lesions could be identified from both maximum-intensity-projection images. Axial CT images revealed small paraaortic lymph node with obviously increased 68Ga-N188 accumulation (SUVmax, 4.3), whereas no abnormal 18F-FDG uptake (SUVmax, 2.2) was noted on corresponding PET/CT images (arrows).
DISCUSSION
The present study serves as a pilot trial of using 68Ga-N188, a radiotracer targeting nectin-4 overexpression, in 16 types of cancers besides UC (7). The positive correlation between membranous nectin-4 expression by IHC and lesion SUVmax by PET/CT was confirmed; this approach may offer a noninvasive evaluation method for the selection of patients suitable for individualized and precision therapy based on EV treatment. Furthermore, a head-to-head comparison of 68Ga-N188 and 18F-FDG was performed to evaluate their detection ability and diagnostic value.
Overexpression of nectin-4 has been reported to be involved in the processes of angiogenesis by upregulating vascular endothelial growth factor, tumor cell proliferation, and metastatic spread by activating the phosphatidylinositol-3 kinase/Akt pathway as well as the epithelial–mesenchymal transition—related signaling pathway (9–12). The level of nectin-4 expression is upregulated in over 90% of UC (13,14), making it a robust biomarker for targeting UC for diagnosis and treatment. EV showed very promising clinical results for treating UC and was recently approved by the U.S. Food and Drug Administration and the European Medical Agency (3,4). Recently, different histologic and molecular subtypes of UC were reported to exhibit marked heterogeneity of nectin-4 expression (2,15–17). Nectin-4 status also varied among different metastases, which resulted in mixed response rates to treatment by EV (6). For example, neuroendocrine and stroma-rich subtypes of UC commonly have lower nectin-4 expression levels than the luminal and basal molecular subtypes (15). Among the nonurothelial histologic types of bladder cancer, only a small proportion of small cell neuroendocrine carcinomas, adenocarcinomas/urachal carcinomas, and squamous cell carcinomas showed high nectin-4 expression (18). Nectin-4 expression has also been observed in multiple cancer types besides UC, including metastatic colorectal, breast, and ovarian cancers; however, the expression levels varied greatly, with considerable heterogeneity (1,2). Those issues create a barrier for selecting patients most likely to respond to EV treatment. A noninvasive nuclear imaging method to quantify nectin-4 expression could be an ideal solution.
Two radiolabeled antibodies for in vivo evaluation of nectin-4 expression have been reported in the preclinical setting. 99mTc-HYNIC-mABNectin-4 for immuno-SPECT imaging showed good performance in the diagnosis of triple-negative breast cancer for guiding nectin-4–targeted treatment (19). AGS-22M6, radiolabeled with 89Zr, was developed as an immuno-PET agent and succeeded in detecting nectin-4 expressing primary tumors as well as liver and bone metastasis in animal models (20). To improve on the pharmacokinetic properties of antibodies and facilitate translational research, a low-molecular-weight PET imaging agent, 68Ga-N188, based on a high-affinity bicyclic peptide scaffold, was recently reported by our group. In a first-in-humans study, it demonstrated a suitable safety profile and pharmacokinetics in 2 healthy volunteers and 14 patients with UC. The sensitivity of 68Ga-N188 in that study was 88.1%, with high specificity, showing the potential to quantitatively image nectin-4 expression (7). We subsequently initiated the study in this article to investigate the imaging properties of 68Ga-N188 in broader cancer types in a head-to-head comparison with 18F-FDG.
In the present study, similar pharmacokinetics were observed for 68Ga-N188 in the 62 patients tested with various cancer types, consistent with our early results for UC (7). We were able to collect 36 tumor samples from the patients and performed IHC staining for broader cancer types. Interestingly, with a sample size larger than that in our previous study of UC (7), the SUVmax of lesions in 68Ga-N188 imaging was found to positively correlate only with nectin-4 membranous, but not cytoplasmic, expression. On the basis of recent findings reported by Klümper et al. (6), several metastatic UC tissues expressed highly heterogeneous levels of membranous nectin-4, a result that highlights the importance of evaluation of nectin-4 expression status before initiation of EV treatment. Decreasing or loss of membranous nectin-4 expression has been observed in a considerable fraction of patients receiving EV treatment, with disease progression, and may indicate an unfavorable response to EV and a poor prognosis. Performing multiple assessments to evaluate the dynamic changes in membranous nectin-4 expression in disease progression over time is important. Although pathologic biopsy could be applied, the status of nectin-4 cannot be adequately reflected by IHC in 1 biopsy, considering the interlesional and intralesional tumor heterogeneity of nectin-4 expression and the possible dynamic changes in expression levels. The findings of positive correlation between 68Ga-N188 imaging and nectin-4 membranous expression confirmed the feasibility of noninvasively and quantitatively accessing tumor burden and nectin-4 status in the whole body using PET imaging. On the basis of the imaging results, we think it might be possible to guide personalized treatment strategies by applying multiple 68Ga-N188 examinations to evaluate membranous nectin-4 expression before and after the initiation of EV treatment.
Similarly, favorable detection rates were found for 68Ga-N188 and 18F-FDG. Compared with 68Ga-N188, 18F-FDG showed higher rates of detection of primary tumors, lymph nodes, and distant metastases, as well as higher sensitivity and accuracy for lymph node involvement. Higher specificity of lymph node metastasis was obtained with 68Ga-N188, although not statistically significant, because of the 18F-FDG avidity for some benign lesions. In the present study, false-positive lesions caused by 18F-FDG avidity in mediastinal and hilar lymph nodes, pulmonary infection, arthritis, and Warthin tumor displayed low 68Ga-N188 activity. Therefore, 68Ga-N188 could be applied as an effective complement to 18F-FDG PET/CT imaging, reducing false-positive results. The SUVmax of 68Ga-N188 in 16 different types of tumors from 62 patients were compared. In UC (n = 16) and pancreatic cancer (n = 19), with relatively large sample sizes, high SUVmax was observed, consistent with previous findings of high levels of nectin-4 expression. As shown in Figure 3, the highest average SUVmax of 68Ga-N188 was for the primary lesions of 2 intrahepatic cholangiocarcinomas. Low background radioactivity in the liver helped to improve the tumor-to-liver ratio, making it possible to detect primary liver tumors and liver metastases clearly.
Six patients with ovarian serous carcinoma showed moderate radioactivity with 68Ga-N188 PET/CT imaging. Discordant nectin-4 expression in different histologies of ovarian cancer has been reported. High nectin-4 expression was seen in about one-half of ovarian serous carcinomas and one-third of ovarian mucinous carcinomas, but only cytoplasmic localization was reported for nectin-4 staining (21); this finding might account for the moderate uptake observed in the present study. Moreover, nectin-4 has been identified as a key gene associated with poor prognosis in high-grade serous ovarian cancers that involve extensive peritoneal involvement, that is, miliary tumors (22). In the present study, the increased and extensive radioactivity distribution related to widespread peritoneal involvement was also noted on 68Ga-N188 imaging (patient 43 in Supplemental Fig. 1), whereas only peritoneal metastatic masses were revealed by 18F-FDG PET/CT imaging.
In the evaluation of residual/recurrent tumors of pancreatic cancer, the rates of detection of 68Ga-N188 and 18F-FDG were 100.00% and 66.67%, respectively. Four residual or recurrent pancreatic tumors were negative on 18F-FDG imaging but positive on 68Ga-N188 imaging. 68Ga-N188 also upstaged the TNM stage in another 2 pancreatic cancer patients with additional lymph node and liver metastases that were missed by 18F-FDG. 68Ga-N188 could be advantageous over 18F-FDG in restaging of pancreatic cancer lesions after neoadjuvant chemotherapy and surgery; this notion still needs to be further confirmed with larger sample sizes and more data. In addition, high levels of nectin-4 expression in pancreatic cancer have been reported to be associated with poor postoperative prognosis and early relapse (23,24). 68Ga-N188 imaging could thus act as a predictor for clinical outcomes in pancreatic cancer. Close follow-up and active surveillance or treatment are reasonable suggestions for pancreatic cancer patients with positive 68Ga-N188 findings. The results of an ongoing follow-up study addressing the application of 68Ga-N188 in pancreatic cancer will be important.
Several limitations of the present study must be acknowledged. First, the small sample size restricted in-depth analysis of 68Ga-N188 performance in multiple cancer types, and current results may be underpowered. The lesion SUVmax may not be representative of cancer types with a limited number of cases. Further studies powered to meaningful endpoints are needed, as are studies that more completely explore the role of 68Ga-N188 as an imaging biomarker. In addition, the relatively short follow-up period of the patients in the present study is not sufficient for further analysis of nectin-4 expression and prognosis.
CONCLUSION
A positive correlation between membranous nectin-4 expression by IHC and SUVmax on nectin-4–targeted 68Ga-N188 PET/CT imaging was observed in a study of 62 patients with 16 different types of cancer, suggesting the potential to apply 68Ga-N188 for selecting patients suitable for individualized and precision EV therapy and monitoring of membranous nectin-4 expression during disease progression. Nectin-4–targeted diagnosis and therapy may be extended to cancer other than UC. A head-to-head comparison of 68Ga-N188 and 18F-FDG was performed in a variety of tumor types. Further investigation is needed to confirm the potential advantages of 68Ga-N188 PET, such as restaging of pancreatic cancer.
DISCLOSURE
This work was funded by the National Natural Science Foundation of China (grants 92059101 and 22277002 to Xing Yang), Beijing Natural Science Foundation (grant Z220014 to Xing Yang), and National High Level Hospital Clinical Research Funding (Scientific and Technologic Achievements Transformation Incubation Guidance Fund Project of Peking University First Hospital grant 2023CX02 to Jianhua Zhang). No other potential conflict of interest relevant to this article was reported.
- © 2024 by the Society of Nuclear Medicine and Molecular Imaging.
REFERENCES
- Received for publication October 9, 2023.
- Revision received January 31, 2024.