Abstract
The use of monoclonal antibodies to deliver radioisotopes directly to tumor cells has become a promising strategy to enhance the antitumor effects of native antibodies. Since the α- and β-particles emitted during the decay of radioisotopes differ in significant ways, proper selection of isotope and antibody combinations is crucial to making radioimmunotherapy a standard therapeutic modality. Because of the short pathlength (50–80 μm) and high linear energy transfer (∼100 keV/μm) of α-emitting radioisotopes, targeted α-particle therapy offers the potential for more specific tumor cell killing with less damage to surrounding normal tissues than β-emitters. These properties make targeted α-particle therapy ideal for the elimination of minimal residual or micrometastatic disease. Radioimmunotherapy using α-emitters such as 213Bi, 211At, and 225Ac has shown activity in several in vitro and in vivo experimental models. Clinical trials have demonstrated the safety, feasibility, and activity of targeted α-particle therapy in the treatment of small-volume and cytoreduced disease. Further advances will require investigation of more potent isotopes, new sources and methods of isotope production, improved chelation techniques, better methods for pharmacokinetic and dosimetric modeling, and new methods of isotope delivery such as pretargeting. Treatment of patients with less-advanced disease and, ultimately, randomized trials comparing targeted α-particle therapy with standard approaches will be required to determine the clinical utility of this approach.
By targeting therapy to specific cell types and disease sites, monoclonal antibodies offer the possibility of decreased toxicity compared with conventional chemotherapy. Although some unlabeled antibodies are capable of inducing major clinical responses, monoclonal antibodies have also been used as delivery vehicles for cytotoxic agents—such as chemotherapy, toxins, and radioisotopes—in an effort to increase their antitumor effects. Despite the predominant use of β-emitters in radioimmunotherapy trials, investigators have long recognized potential advantages of α-particle emitters. α-Particles are positively charged helium nuclei with a shorter range (50–80 μm) and higher energy (5,000–8,000 keV) than β-particles. We review the characteristics of various α-emitting isotopes for the targeted therapy of malignancies, issues in dosimetry, and recent preclinical and clinical studies. The reader is also referred to other published reviews on this subject (1,2).
RADIOBIOLOGY AND RATIONALE FOR α-PARTICLE THERAPY
Although the mechanisms by which radiation induces cell death are not completely understood, several processes have been implicated. Radiation induces single- and double-stranded DNA breaks (3), causes apoptosis (4), and initiates overexpression of p53, leading to delays in the G1 phase of the cell cycle (5). Death of cells exposed to α-particles occurs only when the particles traverse the nucleus; high concentrations of α-particles directed at the cytoplasm have no effect on cell proliferation (6).
Linear energy transfer (LET) and relative biologic effectiveness (RBE) are essential radiobiologic concepts. LET refers to the number of ionizations caused by that radiation per unit of distance traveled. α-Particles have a high LET (approximately 100 keV/μm), whereas, β-particles have a far lower LET (0.2 keV/μm). The RBE for a type of radiation refers to the dose of a reference radiation, usually x-rays, that produces the same biologic effect as the type of radiation in question. The RBE of a type of radiation is in part related to its LET. The RBE of α-particles for cell sterilization ranges from 3 to 7, depending on emission characteristics.
The dependency of RBE on LET can be explained by several differences in the type and extent of cellular damage caused by low- and high-LET radiations. First, high-LET radiation generally causes more irreparable clustered and double-stranded DNA breaks than low-LET radiation (7). The maximum rate of double-stranded DNA breaks occurs at LETs of 100–200 keV/μm, since the distance between ionizations caused by the radiation at these LETs approximates the diameter of double-stranded DNA (2 nm). Second, high-LET radiation causes more severe chromosomal damage, including shattered chromosomes at mitosis and complex chromosomal rearrangements, than low-LET radiation (7). Third, high-LET α-irradiation causes more pronounced G2-phase delays than low-LET γ-irradiation (8). The mechanisms behind these differences in cell cycle effects have not been fully elucidated but may be related to differences in gene expression induced by low- and high-LET radiations (9).
The different physical properties of α- and β-particles confer theoretic advantages and disadvantages to each, depending on the clinical situation. Since the range of β-emissions extends for several millimeters, therapy with isotopes such as 131I, 90Y, and 188Re can create a “crossfire effect,” destroying tumor cells to which the radioimmunoconjugate is not directly bound. In this way, β-emitters can potentially overcome resistance due to antigen-negative tumor cells. Conversely, longer-range β-emissions may also produce nonspecific cytotoxic effects by destroying surrounding normal cells. These characteristics make β-particle therapy better suited for bulky tumors or large-volume disease.
In contrast, α-particles may be better suited to the treatment of microscopic or small-volume disease since their short range and high energies potentially offer more efficient and specific killing of tumor cells. In a microdosimetric model using single-cell conditions, 1 cell-surface decay of the α-emitter 211At resulted in the same degree of cell killing as approximately 1,000 cell-surface decays of the β-emitter 90Y (10). Based on these considerations, α-particle therapy has been investigated in a variety of settings, including leukemias, lymphomas, gliomas, melanoma, and peritoneal carcinomatosis.
SELECTED α-PARTICLE-EMITTING RADIOISOTOPES
Because >100 radioisotopes emit α-particles, and most decay too quickly to be of therapeutic use, we will confine our discussion of α-emitters to those that have therapeutic potential and have been investigated in animal models or humans (Table 1).
Characteristics of Selected α-Emitting Radioisotopes
225Ac
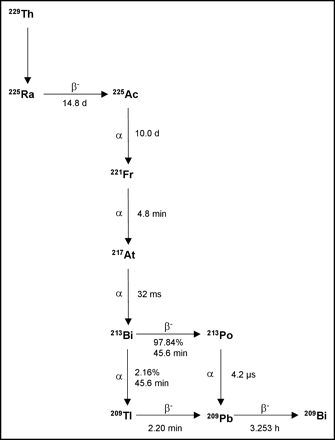
225Ac (half-life, 10.0 d) decays by α-emission through 3 atoms, 221Fr (half-life, 4.8 min), 217At (half-life, 32.3 ms), and 213Bi (half-life, 45.6 min), each of which also emits an α-particle (Fig. 1). 225Ac can be produced by the natural decay of 233U (2) or by accelerator-based methods (11). Because 225Ac radioimmunoconjugates act as atomic nanogenerators, delivering an α-particle cascade to a cancer cell, they are approximately 1,000 times more potent than 213Bi-containing analogs (12). Although this increased potency could make 225Ac more effective than other α-emitters, the possibility of free daughter radioisotopes in circulation after decay of 225Ac raises concerns about the potential toxicity of this radioisotope.
229Th decay scheme. 225Ac is isolated from 229Th sources and decays by α-emission through 221Fr, 217At, and 213Bi, each of which also emits an α-particle.
213Bi
213Bi decays to stable 209Bi by emitting an α-particle and 2 β-particles (Fig. 1). Additionally, a 440-keV photon emission allows detailed biodistribution, pharmacokinetic, and dosimetry studies to be performed. Preparation of 213Bi for clinical use requires a generator consisting of its parent isotope 225Ac dispersed onto a cation-exchange resin from which 213Bi can be eluted (13). After 213Bi is eluted from the generator, the isotope is readily conjugated to antibody molecules appended with the C-functionalized trans-cyclohexyldiethylenetriamine pentaacetic acid moiety, CHX-A-DTPA (14).
212Bi
212Bi (half-life, 60.6 min) emits an α-particle with a mean energy of 7.8 MeV from the decay of 228Th to stable 208Pb. A generator that uses 224Ra as the parent radionuclide facilitates the production of 212Bi for labeling to antibodies (15). 208Tl, produced by the decay of 212Bi, emits a 2.6-MeV γ-ray along with other medium-to-high-energy γ-particles that require heavy shielding to minimize radiation exposure to personnel, thereby limiting the clinical utility of this radioisotope.
223Ra
223Ra (half-life, 11.4 d) can be obtained from uranium mill tailings in large quantities and a generator system has been developed using an 227Ac parent. Like 225Ac, 223Ra emits 4 α-particles over its decay scheme. Because of its bone-seeking properties, unconjugated cationic 223Ra is a promising candidate for delivery of high-LET radiation to cancer cells on bone surfaces. Preliminary results of a clinical phase I study demonstrated pain relief and reduction in tumor marker levels in the treatment of skeletal metastases in patients with prostate and breast cancer (16).
211At
211At (half-life, 7.2 h) decays through a branched pathway with each branch resulting in the production of an α-particle in its decay to stable 207Pb (Fig. 2). The α-particle produced by the decay of 211At has a mean energy of 6.8 MeV and a mean LET of 97–99 keV/μm. Because of its long half-life, 211At-labeled constructs can be used even when the targeting molecule does not gain immediate access to tumor cells. Additionally, its daughter, 211Po, emits K x-rays that allow photon counting of samples and external imaging for biodistribution studies. 211At is produced by the bombardment of bismuth with α-particles in a cyclotron via the 207Bi (α, 2n) 211At nuclear reaction (17) and is isolated from the cyclotron target using a dry distillation procedure (18). Few institutions, however, have a cyclotron capable of producing 211At. Moreover, this radioisotope is retained less well than other α-emitting radiometals after internalization of the antigen-antibody complex (19).
211At decay scheme. 211At decays through a branched pathway with each arm producing an α-particle in its decay to stable 207Pb. EC = electron capture.
RADIOLABELING
The integrity of a radioimmunoconjugates can be susceptible to catabolism after internalization into a target cell or to the direct effects of radioactive decay. Therefore, in vivo stability of a radioconjugate is required to maximize delivery of isotope to tumor and to prevent toxicity. A variety of methods are used to conjugate radioisotopes to antibodies, depending primarily on the nature of the radioisotope. 211At is a halogen, like 131I, and is usually labeled directly to antibodies by incorporation of an aryl carbon-astatine bond into the antibody (17). Methods used to create the aryl carbon-astatine bond usually involve an astatodemetallation reaction using a tin, silicon, or mercury precursor (18,20). Other radioisotopes require bifunctional chelators for linkage to antibodies. Chelators derived from DTPA include the cyclic dianhydride derivative (21) and the cyclohexylbenzyl derivative (CHX-A-DTPA) (14,22). CHX-A-DTPA is effective at chelating bismuth to antibodies, resulting in stable constructs that have been used effectively in clinical trials (14). The macrocyclic ligand 1,4,7,10-tetraazacyclododecane tetraacetic acid (DOTA) and its derivatives have been used effectively for labeling of antibodies with 225Ac. A 2-step procedure was developed in which 225Ac is first conjugated to DOTA-SCN followed by labeling of this construct to antibody (12).
DOSIMETRY
The generally shorter half-lives, shorter range, and higher LET of α-particle emitters distinguishes the dosimetry of these radionuclides from that of β-emitters. For longer-lived isotopes, pharmacokinetics are determined predominantly by the biologic clearance of the antibody. The distribution of the antibody within hours after administration yields residence times that are negligible in proportion to the overall residence times achieved in target and normal organs. In contrast, for 213Bi, 20% of the total α-emissions occur within the first 15 min after injection, and only 6% of the total emissions remain after 3 h, due to its 46-min half-life.
Given the high energy of α-particles delivered over a short range, conventional MIRD methodologies that estimate mean absorbed dose over a specific organ volume may not always yield biologically meaningful information. Because of the physical properties of α-emissions, targeted cells may receive high absorbed-radiation doses, whereas adjacent cells may receive no radiation at all. Therefore, microdosimetric or stochastic analyses that account for the spatial distribution of various cell types and the distribution of α-decays within an organ or region of interest are necessary to estimate the absorbed dose to tumor cells and normal tissues more accurately. Since the geometric relationship between the radionuclide and the target cell is not uniform, α-particle hits cannot be assumed to be a Poisson distribution. Several distributions have been modeled, and microdosimetric spectra expressed as specific energy probability densities have been calculated. Based on this work, methods have been developed to perform basic microdosimetric assessments that account for the probability of the number of hits and the mean specific energy from a single hit (23).
PRECLINICAL AND CLINICAL STUDIES
Mouse Models
The antitumor potential of α-particle radioimmunotherapy was noted in several early studies using xenograft models. Among the first studies, 212Bi-containing radioimmunoconjugates were studied in lymphoma-bearing mice (21) and colon carcinoma-bearing mice (24). Injection of tumor-specific antibodies labeled with 212Bi using cyclic DTPA dianhydride and glycyltyrosyl-lysyl-N-ε-DTPA, respectively, prolonged survival compared with controls. Much of the injected dose of 212Bi, however, was taken up by the kidney, indicating instability of these radioimmunoconjugates (25). To overcome this, a different chelating agent, CHX-A-DTPA, was used to conjugate 212Bi to the anti-gp70 antibody 103A (22). BALB/c mice were inoculated with the Rauscher leukemia virus, resulting in erythroleukemia that expresses glycoprotein gp70. Treatment of the mice with 212Bi-103A resulted in decreased splenic tumor growth and prolonged median survival.
The results of many studies support the hypothesis that α-particle immunotherapy may be more effective in the treatment of small-volume disease than in the treatment of bulky tumors. Administration of 212Bi-anti-Tac (anti-CD25) after inoculation of nude mice with a CD25-expressing plasmacytoma cell line, but before the development of overt disease, led to prolonged tumor-free survival and prevented development of tumors in some animals. In mice with established tumors, however, treatment with 212Bi-anti-Tac failed to produce responses (26). Additionally, in several spheroid models of micrometastatic disease, α-particle therapy has been more effective in reducing the volume of smaller spheroids compared with larger ones (27,28).
In the few studies that have directly compared equivalent doses of α-emitters and β-emitters in animal models, α-emitters have been more effective in inhibiting tumor growth and improving survival rates. In mice with murine ovarian cancer that were treated with 211At-tellurium colloid or with the β-emitters 165Dy, 32P, or 90Y, 211At-tellurium was the most effective and was often curative (29). Similarly, in a human colon cancer xenograft model in nude mice, a 213Bi-labeled Fab′ fragment targeting a glycoprotein found on gastrointestinal cells (CO17-1A) prevented tumor growth and increased survival compared with the controls that received 90Y-labeled Fab′ (30).
213Bi-Labeled Anti-CD33 for Myeloid Leukemia
Based on preclinical data that demonstrated specific tumor killing, safety, and favorable biodistribution in mice (31), a 213Bi-labeled humanized anti-CD33 monoclonal antibody, HuM195, has been studied in clinical trials at Memorial Sloan-Kettering Cancer Center. Eighteen patients with advanced myeloid leukemias were treated in a phase I dose-escalation trial (32). Although myelosuppression occurred in all patients and transient minor liver function abnormalities were common, doses of up to 37 MBq/kg (1 mCi/kg) were safely administered to patients. Within 10 min after injection, γ-camera images demonstrated uptake of 213Bi in the bone marrow, liver, and spleen, without significant uptake in other organs, including the kidney. The absorbed dose ratios between marrow, liver, and spleen and the whole body were 1,000 times greater with 213Bi-HuM195 than with β-emitting HuM195 constructs used in similar patients in previous trials. Fourteen of the 18 patients had a reduction in the percentage of bone marrow blasts after therapy; however, there were no complete remissions, demonstrating the difficulty of targeting 1 or 2 213Bi atoms to each leukemic blast at the specific activities used in this trial (32).
Since α-particle immunotherapy appears to be more useful in the treatment of small-volume disease, a subsequent phase I/II study was undertaken in which patients were first treated with chemotherapy to achieve partial cytoreduction of the leukemic burden followed by 213Bi-HuM195 (33). To date, >20 patients with acute myeloid leukemia were treated with cytarabine (200 mg/m2/d for 5 d) followed by 213Bi-HuM195 at 4 dose levels (18.5–46.25 MBq/kg [0.5–1.25 mCi/kg]). Prolonged myelosuppression was dose limiting at the highest dose level. Complete responses, complete responses with incomplete platelet recovery, and partial responses were seen at the 2 highest dose levels. These preliminary results indicate that sequential administration of cytarabine and 213Bi-HuM195 is safe and can lead to complete remissions in patients with acute myeloid leukemia.
213Bi-Labeled Antibodies Before Nonmyeloablative Transplantation
Although potentially curative for several malignancies, allogeneic marrow transplantation using myeloablative preparative regimens causes significant toxicity. To decrease the toxicity of treatment, nonmyeloablative strategies that rely on a graft-versus-tumor effect have been developed. In an effort to further reduce the toxicity associated with these nonmyeloablative regimens, 213Bi-labeled antibodies to the pan-leukocyte antigen CD45 (34) and the T-cell receptor (TCRαβ) (35) have been used for immunosuppression before marrow transplantation in a canine model. Administration of both radiolabeled constructs before transplantation in conjunction with additional immunosuppressive agents resulted in prompt engraftment of transplanted marrow and resulted in stable mixed chimerism. Toxicities included transient myelosuppression and liver enzyme abnormalities. The use of 213Bi-radioimmunoconjugates for this application in humans may be limited by the high activities (at least 74 MBq/kg [2 mCi/kg]) required to attain engraftment.
225Ac Atomic Nanogenerators
The marked increase in potency of 225Ac constructs over 213Bi-containing analogs in vitro led to studies in nude mice bearing human prostate carcinoma and lymphoma xenografts. Single becquerel (nanocurie) doses of 225Ac-labeled tumor-specific antibodies significantly improved survival over controls and cured a substantial fraction of animals (12). The increased potency of these 225Ac constructs compared with 213Bi can be explained by the longer (10 d) half-life of 225Ac and by the ability of 225Ac conjugates to act as atomic nanogenerators, emitting 4 α-particles within an individual tumor cell as it decays. Based on these promising preclinical data, a phase I trial investigating the use of 225Ac-HuM195 for advanced myeloid leukemias is planned.
211At-Labeled Antibodies
Investigators at Duke University have extensively studied 211At-81C6, a chimeric antibody that targets tenascin, a glycoprotein overexpressed on gliomas relative to normal brain tissue. In a model of neoplastic meningitis, treatment with 211At-81C6 after intrathecal injection of human rhabdomyosarcoma cells prolonged survival compared with controls (36). Based on these data, a phase I dose-escalation trial of 211At-81C6 was initiated in patients with malignant gliomas after surgical resection of the tumor (37). Twelve patients have been treated to date. γ-Camera imaging showed that 99% of the 211At decays occurred within the tumor cavity, indicating high in vivo stability of the radioimmunoconjugate. Early results have suggested that adjuvant therapy with 211At-81C6 prolongs survival in these patients compared with historical controls.
Pretargeting Studies
In an effort to reduce radiation doses to normal organs and improve tumor-to-normal organ dose ratios, pretargeted methods of radioimmunotherapy have been developed. One pretargeting method involves administration of a monoclonal antibody or engineered targeting molecule conjugated to streptavidin, followed by administration of a biotinylated N-acetylgalactosamine-containing “clearing agent” to remove excess circulating antibody. Then, therapeutically radiolabeled biotin is infused. The radiolabeled biotin can bind specifically to “pretargeted” streptavidin at the tumor, whereas unbound radiolabeled biotin is rapidly excreted in the urine (38).
This approach has been applied to a mouse model of adult T-cell leukemia that expresses the cell-surface marker CD25 (39). After treatment with a streptavidin-labeled humanized anti-CD25 antibody and the clearing agent, immunodeficient mice with human adult T-cell leukemia received DOTA-biotin labeled with either 213Bi or 90Y. Treatment with 213Bi reduced the levels of the surrogate tumor markers human β2-microglobulin and soluble CD25 and improved survival compared with controls. Treatment with 90Y, however, did not improve survival compared with controls. Mice treated with 213Bi using the pretargeting approach survived longer than those treated with 213Bi labeled directly to anti-Tac. This approach was also studied using an anti-CD25 single-chain Fv-streptavidin fusion protein followed by radiolabeled biotin (40). Significant antitumor effects were seen after administration of 213Bi-DOTA-biotin to leukemic mice and, when combined with unconjugated anti-Tac, 7 of 10 mice were cured.
CONCLUSION
The role of radiolabeled monoclonal antibodies in the treatment of cancer is increasing. To date, most radioimmunotherapy trials have been performed with β-emitting isotopes. In contrast to β-emitters, the shorter range and higher LET of α-particles allow for more efficient and selective killing of individual tumor cells. Although some experimental models indicate that α-particle immunotherapy may eradicate large tumor burdens, most preclinical and clinical trials suggest that radioimmunotherapy with α-emitters may be best suited for the treatment of small-volume disease. Although results of early studies appear promising, there are several obstacles to the widespread use of α-particle immunotherapy. To address these difficulties, new sources and methods of production of α-emitters, improved chelation chemistry, better pharmacokinetic and dosimetry modeling techniques, and novel delivery methods must be explored. Additional preclinical and clinical investigations are necessary to define optimal radioisotopes, dosing regimens, and therapeutic strategies.
Footnotes
Received Apr. 15, 2004; revision accepted Jun. 11, 2004.
For correspondence or reprints contact: Joseph G. Jurcic, MD, Leukemia Service, Department of Medicine, Memorial Sloan-Kettering Cancer Center, 1275 York Ave., New York, NY 10021.
E-mail: jurcicj{at}mskcc.org