Abstract
242485
Introduction: Cu-mediated radiofluorinations (CMRFs) of arylboronic acids and esters are readily available. Unfortunately, the generation of undesired protodeboronation byporducts and their implications in radiotracer separation negatively affects in vivo binding of radiotracers due to competitive target binding. Therefore, surppressing or avoiding protodeboronation in CMRFs would improve radiotracer quality, patient care and PET imaging outcomes.
Methods: As a starting point, non-radioactive (19F) Cu-mediated nucleophilic fluorinations of 4-biphenylboronic acid as model compound were performed to find the optimal reaction conditions to enhance fluorination while suppressing protodeboronation. Parameters such as solvent, copper source, additives, composition of fluoride labeling cocktail and the reaction temperature were evaluated. The optimized reaction conditions for the 19F-fluorination of 4-biphenylboronic acid using KBF4 (fluoride source), Cu(ONf)2 (copper salt), 18-crown-6 (additive), t-BuOH (solvent) in a 30 minute reaction at 60 °C were used to further establish a radiofluorination protocol for various boronic acids. Radiochemical conversions (RCCs) were determined using radio thin-layer chromatography (ratio-TLC). Radio high-performance liquid chromatography (radio-HPLC) was used to quantify the amount of protodeboronation occurring during these novel reaction conditions and comparing the amount to established methods in the field.
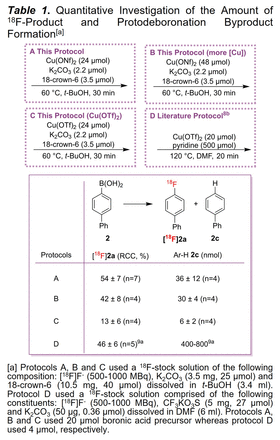
Results: Optimal radiofluorination conditions were established. Fluorine-18 (18F) was trapped using a 130 mg carbonate QMA and eluted using a solution containing K2CO3 and 18‑crown‑6 in MeCN and water (v/v, 8:1) followed by azeotropic drying and re-suspending in anhydrous t-BuOH. Adding this 18F-stock solution to the boronic acid or ester precursor together with Cu(ONf)2 and stirring it for 30 minutes at 60 °C resulted in RCCs of 9-54% (Scheme 1) for a prototypical series of eight (hetero)aryl boronic acids and two (hetero)aryl pinacol-derived boronic esters. With a reliable radiofluorination method in hand, we investigated the amount of protodeboronation produced under these reaction conditions (Table 1, protocol A) using radio-HPLC and a mass calibration curve. Further, the influence on protodeboronation when doubling the amount of Cu(ONf)2 (Table 1, protocol B) as well as switching Cu(ONf)2 to Cu(OTf)2 (Table 1, protocol C) was investigated and lastly compared to an established method from the literature (protocol D). Our protocol A significantly decreased the amount of protodeboronation to 30 nmol in comparison to the literature value of 400-800 nmol (protocol D). Doubling the amount of Cu(ONf)2 (protocol B) did not significantly influence the amount of protodeboronation nor the RCC. Using Cu(OTf)2 instead of Cu(ONf)2 (protocol C) dropped the amount of protodeboronation to a minimum of 6 nmol but at the cost of a significant drop in RCC.
Conclusions: Our modified 18F-labeling protocol successfully suppressed protodeboronation to 30 nmol in comparison to 400-800 nmol stated in the literature while still maintaining good RCCs between 9-54%. Overall, these studies give insight on factors influencing protodeboronation in CMRFs and it is assumed that the suppressed protodeboronation will have a positive effect on PET imaging quality. Higher resolution in PET images will ultimately improve diagnosis and therefore earlier detection and better patient care.