Abstract
242403
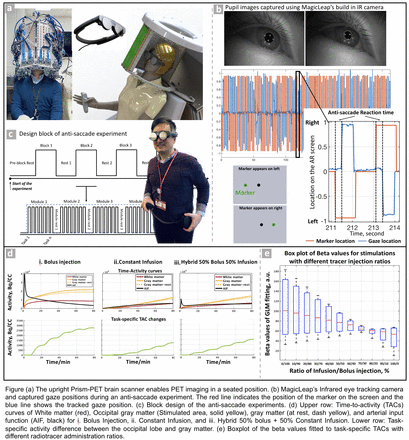
Introduction: Functional [18F]-fluorodeoxyglucose positron emission tomography (FDG-fPET) presents an innovative method to observe the brain's functional activations in response to stimuli and tasks during scanning. Such activation is crucial, as it can amplify the subtle variations in brain functions that might otherwise be obscured in resting-state studies. On the other hand, physiologic signals provide insights into the cognitive processes and functional alterations of specific brain circuits. Integrating fPET with eye tracking and other physiologic signals such as heart rate measurement might provide a robust multi-dimensional framework for understanding brain functions. This is particularly beneficial in the context of complex disorders like Alzheimer’s disease, in which the progression can vary significantly between patients. To fully exploit the benefits of fPET and mitigate its limitations, we propose an ultra-high sensitivity, ultra-high resolution, motion-compensated, portable, and upright Prism-PET brain scanner (Figure a). The proposed scanner will enable fPET imaging in the sitting position with immersive virtual reality (VR) headsets that can provide enhanced behavioral and visual stimulations with infrared eye tracking. EMMT together with Prism-PET can achieve the highest PET motion compensated spatial resolution of ∼1.2 mm and high sensitivity (> 4 times higher than current clinical whole-body PET-CT scanners).
Methods: Eye tracking: We have developed a customized program for MagicLeap AR glasses to perform anti-saccade tasks to capture eye movement and corresponding 2D gaze locations on the screen (Figure b). For the anti-saccade task, participants were asked to look at the mirrored position of where a dot appeared. Anti-saccade accuracy and response speed were derived from the eye tracking data to evaluate the function and performance of the oculomotor and prohibit brain circuits. We also modeled a fPET brain study using a two-compartment kinetic model with K1=0.13, k2=0.16, k3=0.064, and k4=0. Input functions were modeled using two exponential functions. We simulated five 5-minute visual stimulations from 10 to 80 minutes post-injection with 5 minutes rest between tasks. A brief increment of k3 was applied to the occipital region during tasks (Figure d). The task-specific TAC changes were calculated by subtracting the activated gray matter TAC from the resting gray matter TAC. We also modeled the hybrid infusion-bolus injection with different tracer activity injection ratios (from 100% infusion to 100% bolus with a step size of 10%). A total dose of 6 mCi was used. The task-specific TAC curve (Fig. 3, lower row.) was fitted to a general linear model (GLM) with 5 regressors (with each representing one task). The Beta values of the GLM fitting were calculated and the range of betas values for different injection ratios was plotted in Figure e.
Results: Using our customized program, we can output accurate 2D gaze information using MagicLeap with a sampling frequency of 60 Hz. From the fPET simulations, task-specific TAC increment decreases over time for the bolus injection, as the concentration of free in-tissue FDG declines after reaching its peak shortly after the injection. For constant infusion, the task-induced TAC changes were small at the beginning and gradually increased over time. A combination of bolus and constant infusion administration (30% bolus followed by 70% constant infusion) can sustain consistent task-induced dynamic changes in FDG utilization, as the fitted betas values demonstrate a narrow range (Figure e).
Conclusions: The simulation results confirmed that the hybrid bolus-constant infusion can boost the sensitivity and specificity of functional PET imaging by providing. Our simulation results demonstrated that the Prism-PET upright fPET scanner, combined with hybrid injection, can enable sensitive PET functional imaging with high temporal and spatial resolution.